Search API
UCLA Health recently announced it had received a $120 million commitment from surgeon, inventor, and philanthropist Dr. Gary Michelson and his wife, Alya, to kick-start the California Institute for Immunology and Immunotherapy, an innovative public-private partnership aimed at spurring breakthrough discoveries that prevent and cure diseases and catalyze economic growth and innovation in Los Angeles.
Announced on August 27, 2024, the gift will be distributed via the Michelson Medical Research Foundation, designates $100 million to establish two research entities within the institute, each funded by $50 million,
One entity will focus on rapid vaccine development and the other on harnessing the microbiome to advance human health. The microbiome research will be conducted with the new UCLA Goodman-Luskin Microbiome Center.
In addition, the foundation, a part of the Michelson Philanthropies network of foundations, is funding a $20 million endowment to provide research grants to young scientists using novel processes to advance immunotherapy research, human immunology, and vaccine discovery.
“Immunology is the mediator of nearly all human diseases, whether we’re talking about cancer, heart disease, or Alzheimer’s,” Dr. Michelson said in a press release.
“The vision for this institute is to become a ‘field of dreams’ — the world’s leading center for the study of the immune system to develop advanced immunotherapies to prevent, treat, and cure all of the diseases that afflict people today and to end these diseases in our lifetime.
“Scientific research is the key to making possible longer and healthier lives,” Michelson added.

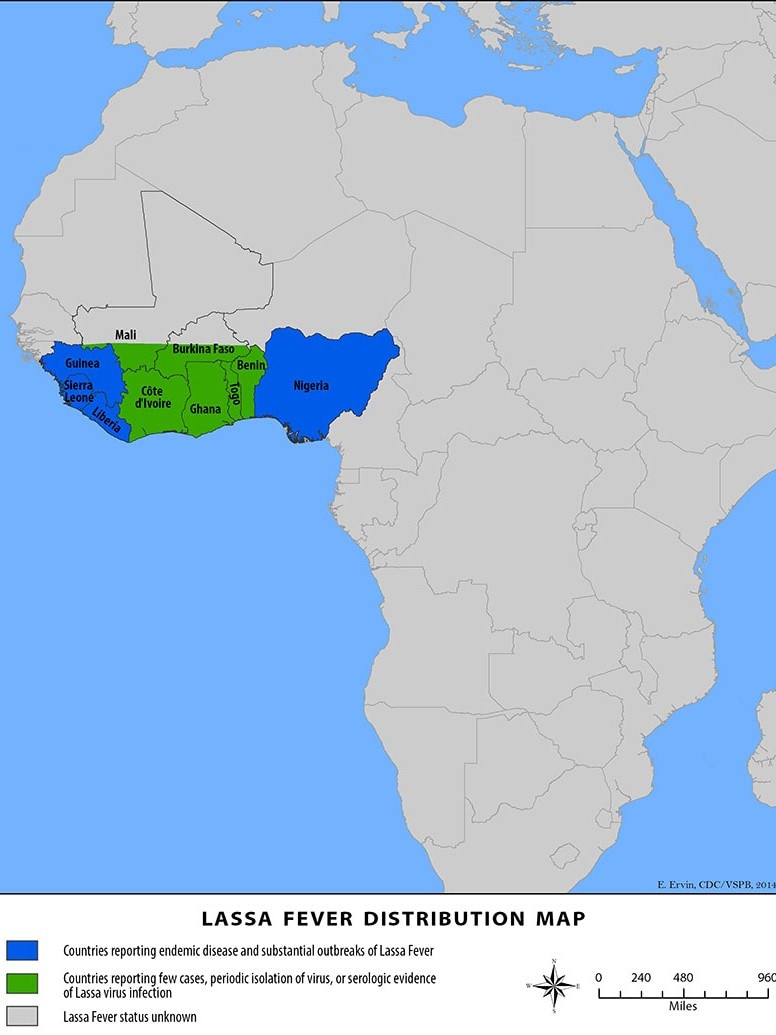
The International AIDS Vaccine Initiative (IAVI) recently announced that clinical trial sites in the Lassa fever-endemic countries of Ghana, Liberia, and Nigeria were vaccinating volunteers in IAVI's C105 study of a Lassa fever vaccine candidate.
This study is designed to evaluate the vaccine candidate’s safety, tolerability, and immunogenicity at two different dosage levels in adults, including people living with HIV, as well as in adolescents and children two years of age and older.
The IAVI C105 study results are expected in 2025. Should the vaccine candidate be found safe and efficacious, IAVI is committed to making its Lassa vaccine affordable and accessible to all needy populations.
As of August 28, 2024, no Lassa fever vaccine currently exists. However, several vaccine candidates are conducting research.
Lassa virus (LASV) is a zoonotic disease that causes the acute viral hemorrhagic illness called Lassa fever, for which treatment is limited.
People can get Lassa fever by contacting infected rats or their saliva, urine, or droppings. The U.S. CDC says that LASV can spread among people.
About 300,000 people fall ill across West Africa annually, though the actual disease burden is thought to be much higher. For these reasons, Lassa fever is featured in the World Health Organization’s R&D Blueprint and requires urgent action due to its potential to cause an outbreak of international concern.
Bharat Biotech International Limited (BBIL) announced on X the launch of HILLCHOL (BBV131), a novel single-strain Oral Cholera Vaccine (OCV).
Like other OCVs, HILLCHOL is a two-dose vaccine, which BBIL says needs to be orally administered on Day 0 and Day 14. The vaccine is suitable for individuals aged above one year.
However, it differs from the other vaccines because it is a single-strain OCV. BBIL stated on August 27, 2024, that this feature enhances manufacturing ease and efficacy.
As of August 2024, there is a global shortage of OCVs.
BBIL's collaboration with MSDInvents, Wellcome Trust, and Hilleman Laboratories aims to address the critical shortage of OCVs.
Currently available OCVs include Valneva SE's DUKORAL®.

Even though we cannot predict what will happen in the United States this upcoming flu season, by examining trends observed in the Southern Hemisphere this past season, we can gain valuable insights into what activity might occur during the forthcoming 2024-2025 Northern Hemisphere flu season.
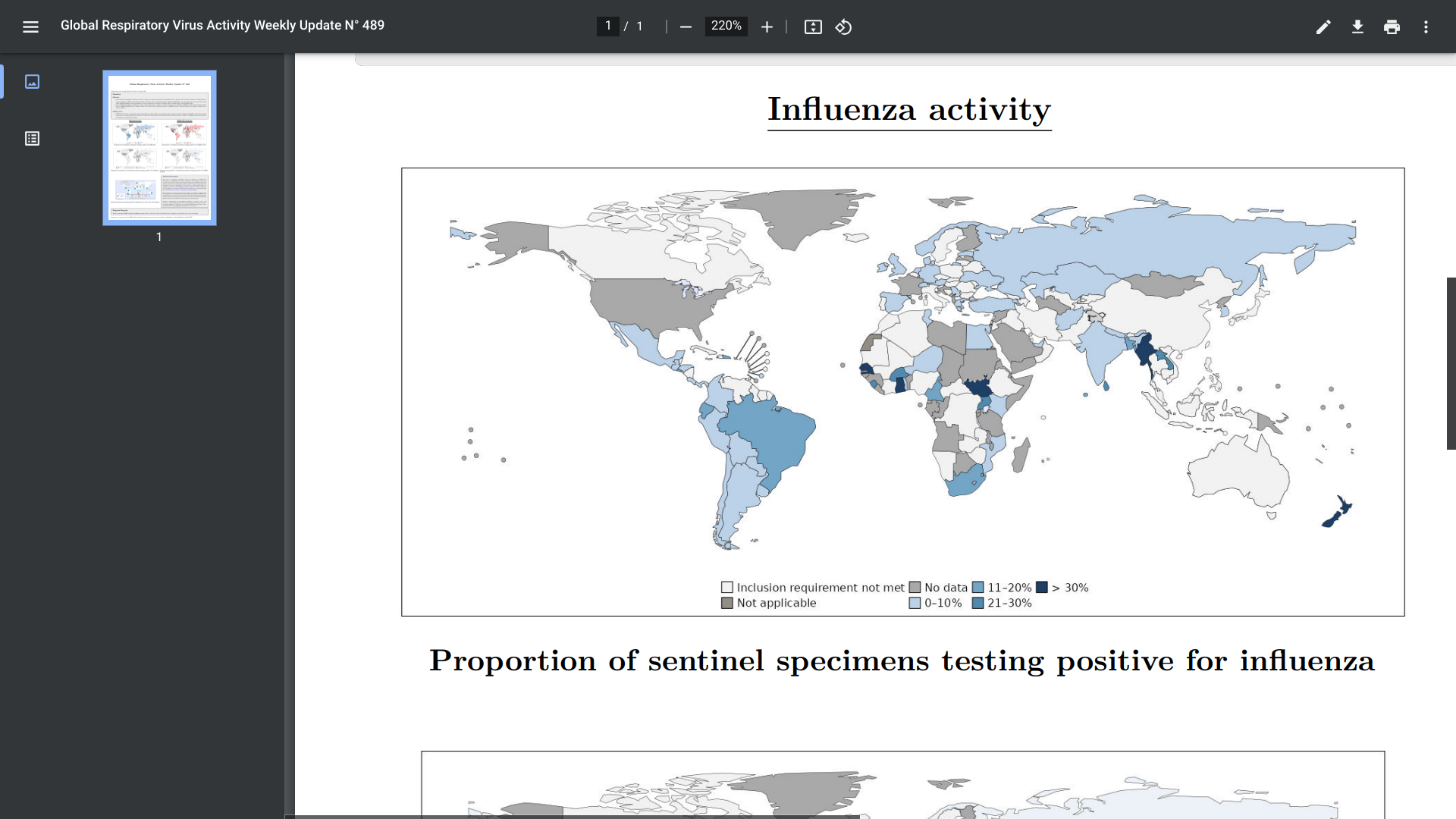
The WHO's Global Respiratory Virus Activity Weekly Update N° 489 was posted on August 11, 2024, confirming in the Southern hemisphere, influenza activity remained elevated in countries in South America (due to influenza A(H3N2) and B viruses), Eastern Africa (due to A(H1N1)pdm09 viruses), Southern Africa (due to B viruses), and Oceania (due to A(H3N2) viruses).
According to the U.S. Centers for Disease Control and Prevention (CDC), on August 26, 2024, during the 2024 Southern Hemisphere flu season, most countries experienced similar levels of flu activity compared to trends observed in prior seasons (2017‒2019 and 2022‒2023 flu seasons).
However, South American and Southern African countries experienced high influenza virus detection levels.
"Vaccination remains the best defense against flu, and even if vaccination does not entirely prevent the risk of flu, it can help reduce the severity of flu illness in people who get flu despite being vaccinated," the CDC wrote.
"In the U.S., September and October are generally good times to be vaccinated against flu."
As of late August 2024, various influenza vaccines are available at health clinics and pharmacies in the U.S.
The U.S. Centers for Disease Control and Prevention (CDC) recently confirmed there is an outbreak of Zika virus in the state of Maharashtra, India.
The Times of India recently reported 113 confirmed Zika cases, of which 100 are from Pune district, including pregnant women.
On August 22, 2024, the CDC published a Level 2 - Practice Enhanced Precautions, Travel Health Notice, offering specific Zika advice.
The CDC says all travelers to Maharashtra should prevent mosquito bites and sexual transmission of the Zika virus during and after travel. Zika virus is most commonly spread to people by the bite of an infected Aedes species mosquito.
If you are planning pregnancy, you should delay pregnancy following travel to India based on the timeframes to prevent sexual transmission.
If you are pregnant, you should avoid travel to Maharashtra. If travel is unavoidable, you should strictly follow Zika prevention recommendations from your healthcare provider.
Infection during pregnancy can cause certain birth defects, says the CDC.
Furthermore, travelers to Maharashtra should seek medical care immediately if they develop fever, rash, headache, joint or muscle pain, or red eyes during or after travel.
In addition to India, there have been over 40,000 Zika cases confirmed in the Region of the Americas in 2024.
There is currently no vaccine to prevent a Zika infection.
However, Valneva SE's VLA1601 second-generation Zika vaccine candidate has progressed in clinical trials.
The journal Clinical Infectious Diseases recently published results from an extensive, observer-blinded, CLOVER phase 3 clinical trial that found a Clostridioides difficile vaccine candidate was safe, well tolerated, and reduced the severity of C difficile infection (CDI).
However, this vaccine did not reduce the incidence of CDI in at-risk adults.
Although the primary endpoint of this study was not met, PF-06425090 reduced symptom duration, CDI requiring medical attention, and CDI-directed antibiotic treatment, highlighting its potential to reduce CDI-associated healthcare burden, wrote these researchers on August 24, 2024.
The U.S. CDC says CDI causes substantial mortality and healthcare burden. In 2022, the incidence rate of CDI increased with age, and rates were higher in women.
As of August 27, 2024, no C difficile infection protection vaccines are available.

CancerVax, Inc. announced today that the Company has recently filed a new patent application, which includes Smart mRNA Technology.
The Company’s new patent application describes a customizable nanoparticle containing Smart mRNA that can DETECT, MARK, and KILL only cancer cells.
By forcing cancer cells to “look” like well-immunized diseases such as measles or chickenpox, we intend to harness the body’s natural immunity to kill cancer cells effectively.
For example, anyone who has had chickenpox, or been vaccinated for chickenpox, has lifetime immunity to the disease. We intend to activate and harness this natural immunity to fight cancer.
Dr. Adam Grant, Principal Scientist at CancerVax and co-inventor of this new technology commented in a press release on August 27, 2024, “In the creation of this new technology, we have been using cutting-edge machine learning and artificial intelligence algorithms to identify genetic signatures that differentiate cancer cells from healthy cells."
"We can quickly load these signatures into our Smart mRNA for immediate lab testing. Not only does this speed up innovation, but it drastically reduces the current laborious and iterative drug discovery process. Only recently have the scientific tools and data been available to allow us to discover and innovate this technology."
"As a computational biologist by training, I have watched the advancement of drug delivery systems over the years, and the availability of next-generation sequencing data sets grow to the point where we can now design new and exciting drugs that we believe can change the game in cancer treatments.”
Dr. Grant continued, “Our natural immune system is an expert at identifying and eradicating foreign pathogens. When a pathogen is eliminated from the body, our immune system remembers it if it infects the body again."
"Our Universal Cancer Treatment Platform harnesses this extraordinary immune system capability by marking cancer cells with something the immune system already knows, such as measles. This way, the immune system can easily kill the cancer cells, just as it would with measles."
"In contrast to other cancer therapies, our technology employs the full power of the body’s immune system. It has the potential to turn “cold” tumors into “hot” tumors, overcoming a major hurdle in treating cancer patients with existing immunotherapies."
We look forward to validating our hypotheses using in-vivo models and refining our technology shortly.”