Search API
New research by modeling experts shows that vaccinating against Lassa Fever—a viral disease—would prevent millions of people from falling ill.
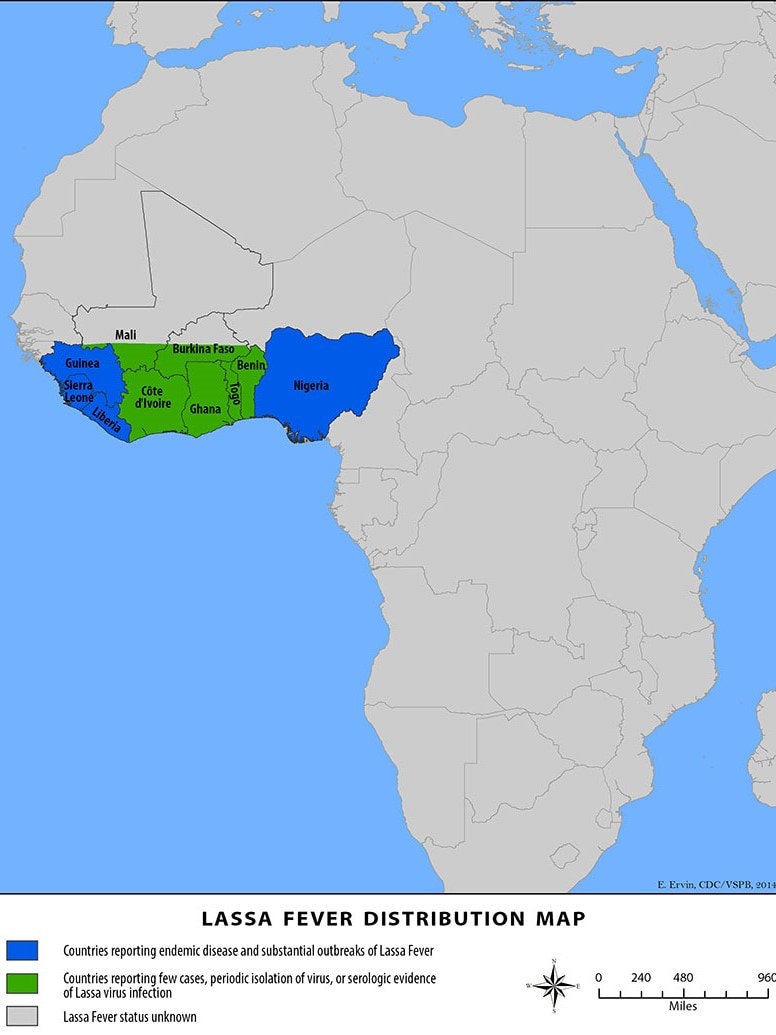
Lassa fever is found in parts of West Africa, including Sierra Leone, Liberia, Guinea, and Nigeria, where the first documented case occurred in 1969.
According to modeling by the Universities of Oxford and Liverpool and the Liverpool School of Tropical Medicine, deploying an effective Lassa vaccine across 15 countries of continental West Africa could save nearly 3,300 lives over ten years, wrote the Coalition for Epidemic Preparedness Innovations (CEPI).
The model was published on August 28, 2024, and predicted 2.7 million (95% uncertainty interval: 2.1–3.4 million) Lassa virus infections annually. However, due to limited access to diagnostics and healthcare, Lassa’s true disease burden could be much higher than reported.
They also model the emergence of ‘Lassa-X’—a hypothetical pandemic Lassa virus variant—and project impacts of achieving 100 Days Mission vaccination targets.
The results showed that around 5,500 lives could be saved and 33,000 hospitalizations avoided throughout a two-year outbreak if safe and 70% effective Lassa X vaccines were given to 40% of people per year starting within 100 days.
Overall, the most effective vaccination strategy was a population-wide preventive campaign targeting WHO-classified ‘endemic’ districts.
Richard Hatchett, CEO of CEPI, commented in a press release, “This study demonstrates the urgent need for a vaccine to protect people from this debilitating and sometimes deadly disease. Lassa fever has been a priority for CEPI since our launch in 2017, and we are proud to be one of the world’s leading Lassa vaccine R&D funders.”
CEPI is one of the world’s leading Lassa vaccine candidate R&D funders. To date, it has invested in six potential vaccine candidates, of which four have progressed into human testing.
One of CEPI’s partners, IAVI, has launched the first-ever Phase II clinical trial of a Lassa vaccine in Abuja, Nigeria.
In the northeastern section of the United States, concerns about the impact of Eastern equine encephalitis (EEE) and West Nile virus (WNV) have continued into September 2024.
The Massachusetts Department of Public Health (DPH) announced on September 5, 2024, one additional human case of EEE and one additional human case of WNV in Massachusetts this year.
The total number of EEE cases in Massachusetts this year is three, with seven WNV cases.
WNV risk levels in the following communities are being raised to high: Stoneham and Wakefield in Middlesex County.
Throughout the U.S., 38 states have reported WNV cases in 2024.
The last outbreak of EEE occurred in 2019-2020 and resulted in 17 human cases and seven deaths. In 2023, there were six human cases of WNV.
As of September, EEE risk levels have been raised to high in Acton, Ayer, Boxborough, Carlisle, Littleton in Middlesex County, and Harvard in Worcester County. The following communities are being raised to moderate: Bedford, Billerica, Chelmsford, Concord, Framingham, Groton, Lincoln, Shirley, Stow, Tyngsborough, Wayland, and Westford in Middlesex County; and Berlin, Bolton, Clinton, and Lancaster in Worcester County.
Public Health Commissioner Robbie Goldstein, MD, PhD., commented in a press release, “It is essential that residents continue to use mosquito repellent with an EPA-registered active ingredient every time they are outdoors. We also strongly recommend that residents and towns in high-risk areas for EEE reschedule their evening outdoor events to avoid peak mosquito biting hours.”
EEE and WNV are transmitted to humans through the bite of an infected mosquito.
“Mosquito behavior starts to change in September,” said State Epidemiologist Dr. Catherine Brown. “They will be less active during cooler temperatures. However, during warmer weather, such as being forecast for the end of next week, mosquitoes will be out and looking for their next meal.”
To the north of Massachusetts, the New Hampshire Department of Health and Human Services reported on September 4, 2024, that ten mosquito batches tested positive for EEE this year, with one fatal human case.
The last reported human cases of EEE in New Hampshire were in 2014 when three cases were identified. Two of those patients died.
As of September 6, 2024, no vaccines are available for people to protect themselves against EEE or WNV.
Novavax Inc. announced on X today that its updated 2024-2025 formula COVID-19 vaccine (NVX-CoV2705) has received Marketing Approval from Japan’s Ministry of Health, Labour and Welfare.
As of September 6, 2024, Novavax's vaccine is the only non-mRNA, protein-based COVID-19 vaccine available in the U.S. following the Food and Drug Administration (FDA) granting emergency use authorization.
John C. Jacobs, President, and Chief Executive Officer, Novavax, commented in a recent press release, "Our updated vaccine targets JN.1, the 'parent strain' of currently circulating variants, and has shown robust cross-reactivity against JN.1 lineage viruses, including KP.2.3, KP.3, KP.3.1.1 and LB.1."
Since August 2020, Novavax and Takeda Pharmaceutical Company Limited have partnered to develop, manufacture, and commercialize Novavax’s COVID‑19 vaccines.
Before visiting Japan, the U.S. CDC recommends travelers speak with a healthcare provider one month before departure about their vaccination options.
During the global dengue virus outbreak in 2024, Puerto Rico was the most impacted area in the United States. The U.S. CDC says that Dengue is endemic in Puerto Rico, cases are increasing, and it issued a Dengue travel advisory earlier in 2024.
As of week #32 in August 2024, Puerto Rico reported 2,704 dengue cases, with San Juan reporting over 950 cases.
The local health department's report for August 2024 indicates that 1,234 dengue-related hospitalizations and two dengue-related deaths occurred this year.
Last year, 1,242 dengue cases were reported in Puerto Rico.
Since 2019, the U.S. Food and Drug Administration (FDA) has approved the Dengvaxia vaccine for certain people with laboratory evidence of a previous dengue infection and living in areas where Dengue is endemic, such as Puerto Rico.
However, in late August 2024, Puerto Rico's Health Department issued a Notice of Discontinuation regarding access to Dengvaxia.
This announcement indicates that millions of visitors to Puerto Rico in 2024 will be unable to access a Dengue-preventive vaccine.
The FDA may approve various dengue vaccines in the coming months and once again become available throughout the U.S.
Additionally, diseases such as Chikungunya have been transmitted to people by the Aedes aeqypti mosquito.
The good news is that Valneva SE's IXCHIQ® single-dose chikungunya vaccine is available in the U.S. at travel clinics and pharmacies.

When the U.S. Centers for Disease Control and Prevention (CDC) recommended respiratory syncytial virus (RSV) vaccination for seniors in 2023, prelicensure trials were not powered to assess efficacy against RSV-associated hospitalization, excluded immunocompromised patients, and underrepresented other groups at increased risk of severe RSV disease, including adults aged 75 years and older.
A recent real-world study published by The JAMA Network Research Letter on September 4, 2024, evaluated the effectiveness (VE) of the RSV vaccine against RSV-associated hospitalization during the first season of use.
These researchers concluded VE against RSV-associated hospitalization was 75% (95% confidence interval (50% to 87%).
As of May 2024, the CDC RSVVaxView estimated that 24.4% of adults 60 and older received an RSV vaccine.
As of September 2024, three RSV vaccines are authorized for use in the United States and are readily available at most clinics and pharmacies before the 2024-2025 RSV season intensifies during the winter months.

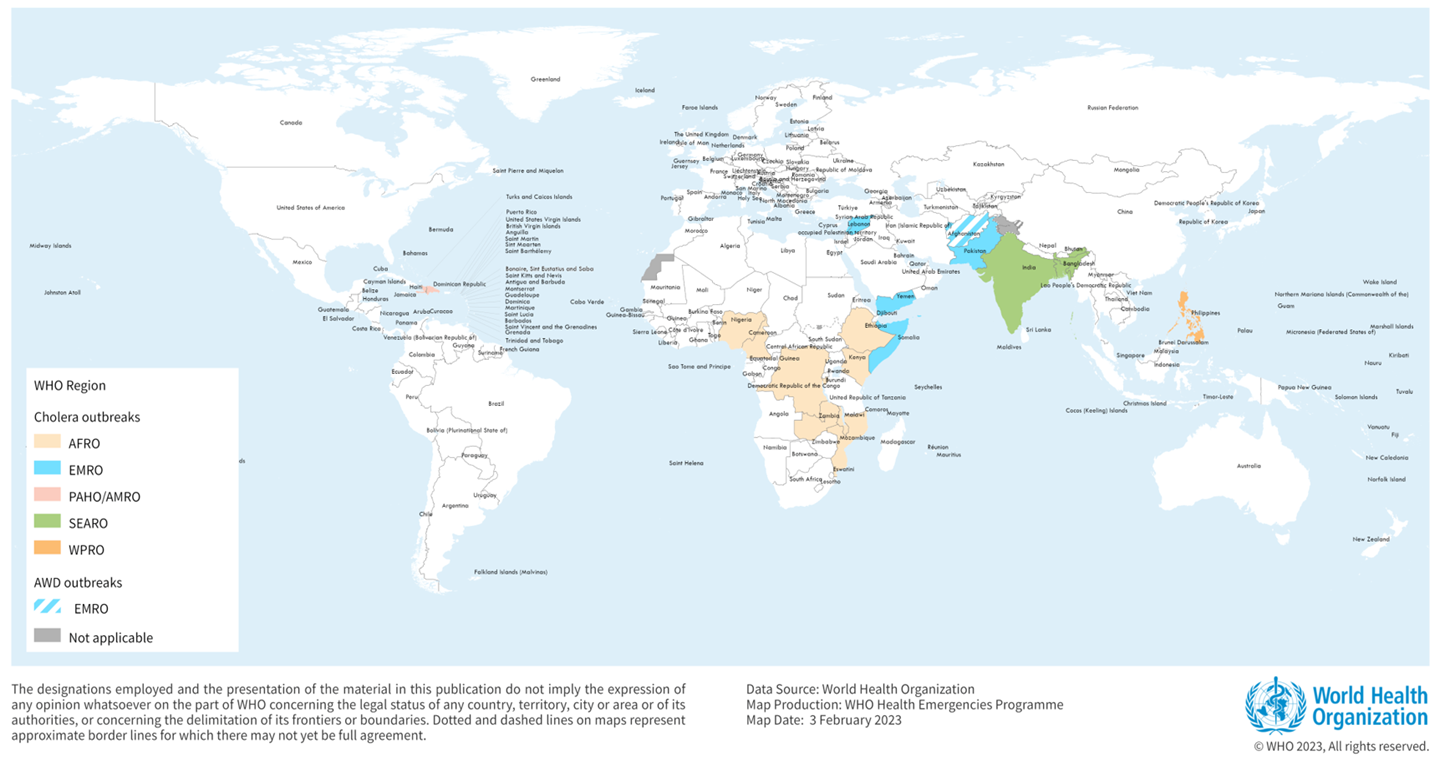
The World Health Organization (WHO) announced today it considers the current global risk from cholera as very high since cholera cases increased by 13% and deaths (~4,000) by 71% last year compared to 2022.
Across all continents as of mid-August 2024, 342,800 cholera cases and 2,400 related deaths have already been reported to WHO.
Unfortunately, as of September 4, 2024, there is insufficient oral cholera vaccine (OCV) supply to meet the global demand.
Since October 2022, the International Coordinating Group, which manages emergency vaccine supplies, has suspended the standard two-dose vaccination regimen in cholera outbreak response campaigns, adopting a single-dose approach instead to reach and protect more people given limited supplies.
Despite the low stockpile of OCV, a record 35 million doses were shipped last year, with the one-dose strategy in effect.
The WHO has prequalified Dukoral®, Shanchol™, Euvichol®, and the Euvichol-S OCVs to address the supply shortfall.
In the United States, OCVs are available at travel clinics and pharmacies.
Without a herpes preventive vaccine available in 2024, post-infection treatments offer patients their best option.
Over the past few years, an antiviral drug named Acyclovir has been used to slow the spreading and lessen the symptoms of the Herpes Simplex Virus 1 (HSV-1) virus.
However, Acyclovir will not cure herpes.
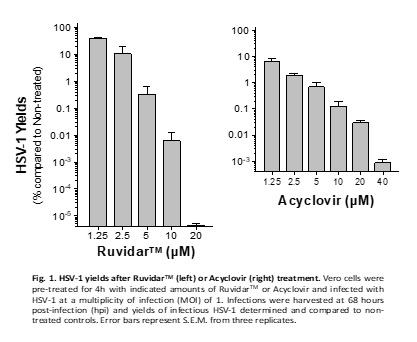
To address this need, Theralase® Technologies Inc. today announced that its lead drug formulation, Ruvidar™, could be more effective in destroying HSV-1 than Acyclovir.
Another important pre-clinical observation is that Acyclovir could not prevent HSV-1 replication if added one day after infection. However, Ruvidar prevented HSV-1 replication by ten million-fold when added one day after infection.
In other words, from a clinical perspective, if a patient has pre-existing HSV-1, then Acyclovir could not prevent the virus' replication; however, Ruvidar TM would be highly effective, the company wrote on September 3, 2024.
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase, stated in a press release, “This latest research continues to strengthen what we already know; Ruvidar is a very potent drug in the destruction of cancer, viruses, and bacteria on its own and is further enhanced by light, radiation, sound or drug activation."
"Based on this latest research, Theralase® plans to commence seeking a partner/licensing opportunity in the development of Ruvidar™ for both a topical and oral treatment for the prevention and treatment of herpes simplex.“
In previous research, Ruvidar was found effective at inactivating both enveloped and non-enveloped viruses.
Vaxcyte, Inc. today announced that it has commenced an underwritten public offering of $1.0 billion of its common stock and pre-funded warrants.
As of August 3, 2024, Vaxcyte intends to grant the funding underwriters a 30-day option to purchase up to an additional $150 million of shares of its common stock offered in the public offering (including shares underlying the pre-funded warrants).
Vaxcyrte confirmed this offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed or as to the actual size or terms of the offering.
The Company is developing broad-spectrum conjugate and novel protein vaccines to prevent or treat bacterial infectious diseases.
VAX-31 is a Phase 3-ready 31-valent, carrier-sparing pneumococcal conjugate vaccine (PCV) candidate being developed for the prevention of invasive pneumococcal disease (IPD) in adults and infants and is the broadest-spectrum PCV candidate in the clinic today.
VAX-24, the Company’s 24-valent PCV candidate, is designed to cover more serotypes than any infant PCV on-market and is currently being evaluated in a Phase 2 infant study.
Both VAX-31 and VAX-24 are designed to improve upon the standard-of-care PCVs by covering the serotypes in circulation that are responsible for a significant portion of IPD and are associated with high case-fatality rates, antibiotic resistance, and meningitis while maintaining coverage of previously circulating strains that are currently contained through continued vaccination practice.