Search API
According to media reporting, a student in southern India has died after being infected with the Nipah virus. As of September 14, 2024, 151 people in contact with the student are now being monitored.
India reported its last fatal Nipah case in July 2024.
According to the World Helath Organization (WHO), the first Nipah virus disease outbreak in India was reported in Siliguri in 2001. State authorities alerted Mysuru, Mangaluru, Chamarajanagar, and Kodagu districts in Karnataka, which borders Kerala state.
Currently, the WHO and other health agencies have not issued travel advisories regarding Nipah outbreaks.
The WHO says Nipah has a relatively high case-fatality ratio and is an emerging zoonotic disease of public health importance in the South East Asia and Western Pacific WHO Regions. It was first identified during an outbreak in Malaysia in 1998.
In June 2023, the Coalition for Epidemic Preparedness Innovations invested up to U.S. $100 million in four Nipah vaccine candidate projects. These candidates include live-attenuated and replication-defective recombinant vaccine platforms based on poxviruses, VSV, adenovirus, measles, rabies, and virus-like particles and subunit vaccines.
As of September 17, 2024, neither the U.S. Food and Drug Administration nor the European Medicines Agency has authorized a vaccine candidate for the Nipah virus, but clinical trials are ongoing.
With over 723 deaths from different mpox outbreaks in 14 countries of the African Region, the World Health Organization (WHO) has expanded access to one mpox vaccine.
On September 13, 2024, the WHO announced Bavarian Nordic A/S's MVA-BN (JYNNEOS®) vaccine is the first vaccine against mpox to be added to its prequalification list.
“The WHO prequalification of the MVA-BN vaccine will help accelerate ongoing procurement of the mpox vaccines by governments and international agencies such as Gavi and Unicef to help communities on the frontlines of the ongoing emergency in Africa and beyond,” said Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, in a press release.
While MVA-BN is currently not licensed for persons under 18, this vaccine may be used “off-label” in infants, children, adolescents, pregnant women, and immunocompromised people. This means vaccine use is recommended in outbreak settings where the benefits of vaccination outweigh the potential risks.
Additionally, the WHO also recommends single-dose use in supply-constrained outbreak situations.
Dr. Rogerio Gaspar, WHO Director for Regulation and Prequalification. “We are progressing with prequalification and emergency use listing procedures with manufacturers of two other mpox vaccines: LC-16 and ACAM2000."
The escalating mpox clade 1 outbreak in the Democratic Republic of the Congo and other countries was declared an emergency by the WHO Director-General on August 14, 2024.
As of mid-Agust 2024, the United States has not reported mpox clade 1 cases, but the JYNNEOS vaccine is commercially available at select clinics and pharmacies.

Albert Einstein College of Medicine recently announced it received a five-year, $14 million per year grant from the National Institute of Allergy and Infectious Diseases (NIAID) to participate in a broad national effort to develop "plug-and-play" vaccines and antibody-based therapies against a wide range of emerging viruses.
The Einstein-led consortium, called PROVIDENT (Prepositioning Optimized Strategies for Vaccines and Immunotherapeutics Against Diverse Emerging Infectious Threats), will link 13 teams in academia, government, and industry that will conduct four projects designed to:
Discover and analyze virus-host interactions and the molecular mechanisms involved in viral disease,
Design proteins to elicit antiviral immune responses and then evaluate and optimize those responses,
Create “road maps” for quickly developing RNA vaccines against microbes with pandemic potential, and
Map the antibody responses observed in people infected with viruses and use this knowledge to design vaccines and therapeutics.
PROVIDENT builds on NIAID’s 2021 Pandemic Preparedness Plan, which focuses on “priority pathogens” and “prototype pathogens.” Priority pathogens include viruses known to cause significant human illness or death, such as dengue and Ebola.
“Recent outbreaks of mpox, Nipah virus, and Eastern equine encephalitis, among other viral infections, underscore the need for an even broader preparedness program,” said Eva Mittler, Ph.D., research assistant professor at Einstein and leader of one of the PROVIDENT components, in a press release on September 13, 2024.
“We don't know what virus will cause the next pandemic.”
The $70 million grant is part of NIAID’s new Research and Development of Vaccines and Monoclonal Antibodies for Pandemic Preparedness (ReVAMPP) Network.
The ReVAMPP network focuses on viruses from the Flaviviridae family, which features viruses that cause dengue and yellow fever; the Paramyxoviridae family, which contains viruses that cause measles, mumps, and Nipah-induced encephalitis; the Picornaviridae family, whose members cause poliomyelitis, foot-and-mouth disease, and myocarditis; the Togaviridae family, which contains viruses that induce Chikungunya virus-induced arthralgia or encephalitis and Venezuelan equine encephalitis; as well as viruses from 5 different families within the Bunyavirales order, including Sin Nombre virus from the Hantaviridae family and the viruses that cause Rift Valley Fever (Phenuiviridae), Crimean Congo Hemorrhagic Fever (Nairoviridae), Oropouche Fever (Peribunyaviridae), and Lassa Fever (Arenaviridae).
Bavarian Nordic today announced the commercial availability of Vaxchora® (CVD 103-HgR), the only single-dose oral vaccine approved in Canada to protect against cholera.
For approximately 10% of people infected with cholera, a sudden and severe intestinal infection marked by acute watery diarrhea, a severe presentation of cholera can rapidly cause dehydration and death if left untreated.
Nearly 4 million cases of cholera occur during outbreaks annually. The number of reported cholera-related deaths increased by 71% in 2023 compared to 2022.
“To help (Canadians) prevent illness while traveling internationally, we are expanding our vaccine offerings to include protection against cholera. Healthcare providers can now offer this new vaccine option to travelers who plan to visit countries where cholera is present,” said Karinne Lacombe, Canada Country Director, Bavarian Nordic, in a press release on September 16, 2024.
Cholera is endemic to approximately 50 countries and is common in Asia, Africa, and Central and South America. The Southeast Asia region, which includes Bangladesh and India, has the largest populations at risk for cholera.
While cholera is most commonly transmitted by consuming contaminated water, it may also be acquired from eating raw or undercooked food, especially fish and shellfish.
Other cholera vaccines, such as Valneva SE's oral, inactivated DUKORAL® vaccine, will be available globally in 2024. According to the World Health Organization, two doses of Dukoral® provide protection against cholera for two years.
Most cholera cases in the United States are linked to international travel, such as to Haiti.
Travel vaccine experts offer cholera vaccination advice at clinics and pharmacies in the U.S.
As the respiratory syncytial virus (RSV) activity ramps up for the 2024-2025 season, more children will have access to an innovative therapy this year.
Sanofi U.S. announced today that it is shipping BEYFORTUS™ 50mg and 100mg injection doses to private healthcare providers in the United States.
BEYFORTUS (Nirsevimab-alip) is the first and only long-acting monoclonal antibody approved for the prevention of RSV lower respiratory tract disease (LRTD) in newborns and infants born during or entering their first RSV season and for children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
BEYFORTUS is not an RSV vaccine but offers passive immunization.
Bridgewater, N.J.-based Sanofi stated it anticipates having enough supply so that every eligible baby born outside of the season will have access to immunization at a regular checkup, and those born during the season will have access at birth.
Thomas Grenier, Head of Vaccines, North America, Sanofi, commented in a press release on September 16, 2024, "We're proud to offer BEYFORTUS doses to help protect every eligible baby in the U.S. this RSV season."
"This upcoming season, we look forward to BEYFORTUS offering its demonstrated real-world protection to as many infants as possible."
Sanofi also took additional measures to ensure greater readiness for this season by launching the BEYFORTUS Reservation Program, which provides critical insight into private healthcare provider demand and allows for prioritized fulfillment of requests placed through the program.
Furthermore, the Centers for Disease Control and Prevention's Vaccines for Children program will help ensure that the majority of doses are available before RSV season.
As of May 2024, about 41% of women with young infants reported that their child had received BEYFORTUS.
This announcement is essential as RSV is a highly contagious virus that can lead to serious respiratory illness in infants. Two out of three infants are infected with RSV during their first year of life, and almost all children are infected by their second birthday.

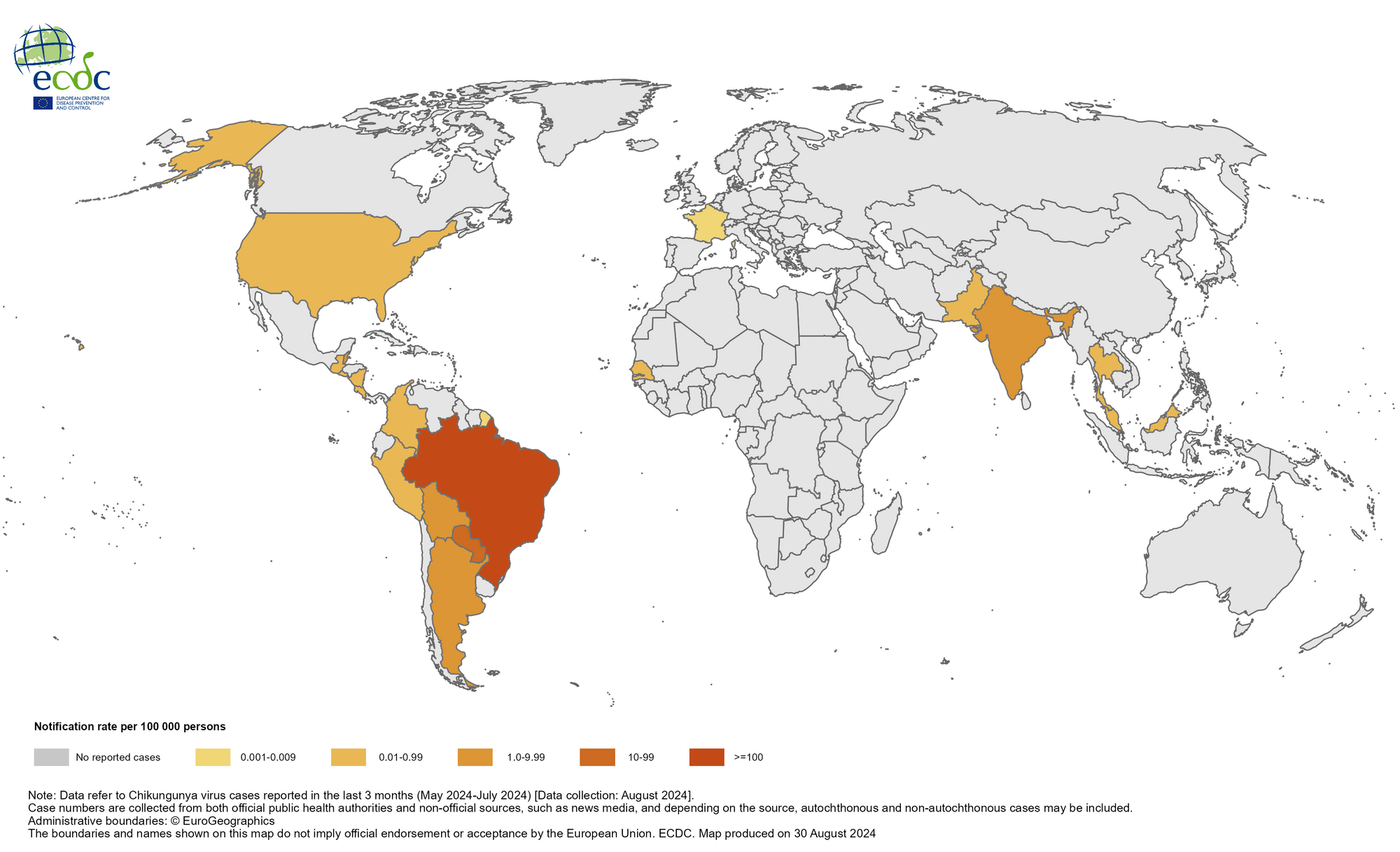
The Chikungunya virus (CHIKV) disease was first reported in the Republic of India in 1963 and has since spread to all parts of the subcontinent, becoming endemic. This health risk is seldom discussed, with over 9 million people visiting India, including about 1.5 million people from the United States.
As of early September 2024, India's health department had reported 69,395 cases of CHIKV this year. At this rate, India may exceed last year's unfortunate outbreak record of 200,064 CHIKV cases, which was the most significant number reported in Asia.
For example, the Times of India reported on September 14, 2024, that chikungunya cases had spiked in Maharashtra. By September 7, 2024, there were 2,643 cases, surpassing the 1,702 recorded in 2023, representing a 55% increase in just nine months.
This year, Pakistan (1,302), The Maldives (389), and Thailand (280) have also reported increases in CHIKV cases in the Asia/Pacific region.
As of early August 2024, approximately 450,000 CHIKV cases and over 160 deaths have been reported worldwide.
To alert international travelers, the U.S. CDC recently stated that the Chikungunya vaccination (Valneva SE's IXCHIQ®) may be considered for certain visitors and long-term residents in 2024.
The CDC also recommended long-term visitors to India consider Japanese encephalitis vaccination (IXIARO® JESPECT®).
These vaccines are generally available at travel clinics and pharmacies in the U.S. and should be administered at least one month before traveling abroad.
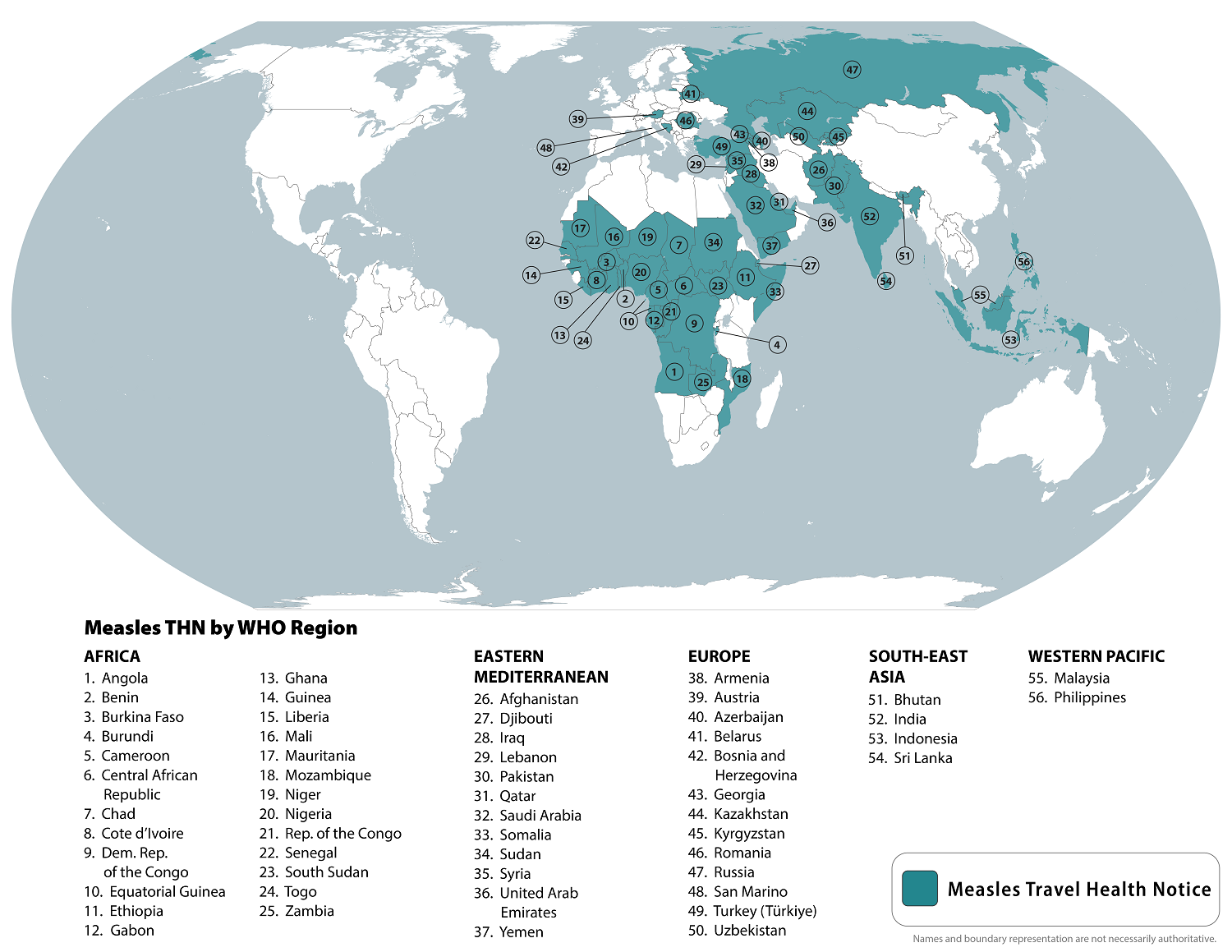
As the global measles outbreak continues in 2024, a 32nd U.S. state confirmed its initial case.
The Tennessee Department of Health (TDH) recently confirmed that a Tennessee resident who recently traveled internationally is recovering from a measles infection.
As of September 14, 2024, TDH has not identified additional measles cases in Tennessee. The last year in which TDH reported positive measles cases in Tennessee was 2019.
On September 5, 2024, the U.S. CDC reported 251 measles cases in 31 jurisdictions this year. So far this year, Chicago (61), Minneapolis (49), and Portland (31) have reported an unusual number of cases.
The measles virus can spread through the air when an infected person speaks, coughs, or sneezes. It can live for up to two hours in the air or on a surface.
Symptoms include fever, headache, and general unwellness, followed by fever, rash, cough, red eyes, or congestion, says the TDH.
To notify international travelers of the measles outbreaks, the CDC reissued a Level Travel Health Advisory in August 2024, identifying 56 countries reporting measles cases to alert international travelers of the ongoing health risk.
The CDC recommends that international travelers speak with a healthcare provider at least one month before traveling abroad about measles vaccination options.
CSL and self-amplifying mRNA (sa-mRNA) pioneer Arcturus Therapeutics today announced that Japan's Ministry of Health, Labor and Welfare (MHLW) granted approval and authorization for their updated sa-mRNA COVID-19 vaccine, KOSTAIVE®.
According to the September 13, 2024, press release, KOSTAIVE is the world's first commercially available sa-mRNA COVID-19 vaccine for adults 18 and older.
Unlike conventional mRNA vaccines, sa-mRNA vaccines instruct the body to make more mRNA and protein to boost the immune response.
"We believe KOSTAIVE® has the potential to change the paradigm for COVID-19 vaccines in Japan," commented Jonathan Edelman, M.D., Senior Vice President, Vaccines Innovation Unit, CSL.
"Today's approval further demonstrates CSL's promise to pursue, develop, and deliver new innovative treatment options to protect public health."
Meiji Seika Pharma, CSL's exclusive partner in Japan, will begin vaccine distribution next month.
Following a peak in July 2024, MHLW data indicates COVID-19 cases in Japan have rapidly declined as of early September.
To avoid diseases such as Japanese encephalitis (IXIARO®), measles, and rubella, the U.S. CDC recommends speaking with a travel vaccine expert at least one month before visiting Japan.
CSL, including three businesses: CSL Behring, CSL Seqirus, and CSL Vifor, provides lifesaving products to patients in more than 100 countries and employs 32,000 people.