Search API
PharmaJet® today announced that the journal Nature published results from Gennova Biopharmaceutical's Phase 2/3 clinical trial. The trial was conducted to evaluate the safety and immunogenicity of its novel samRNA-based Covid-19 vaccine booster.
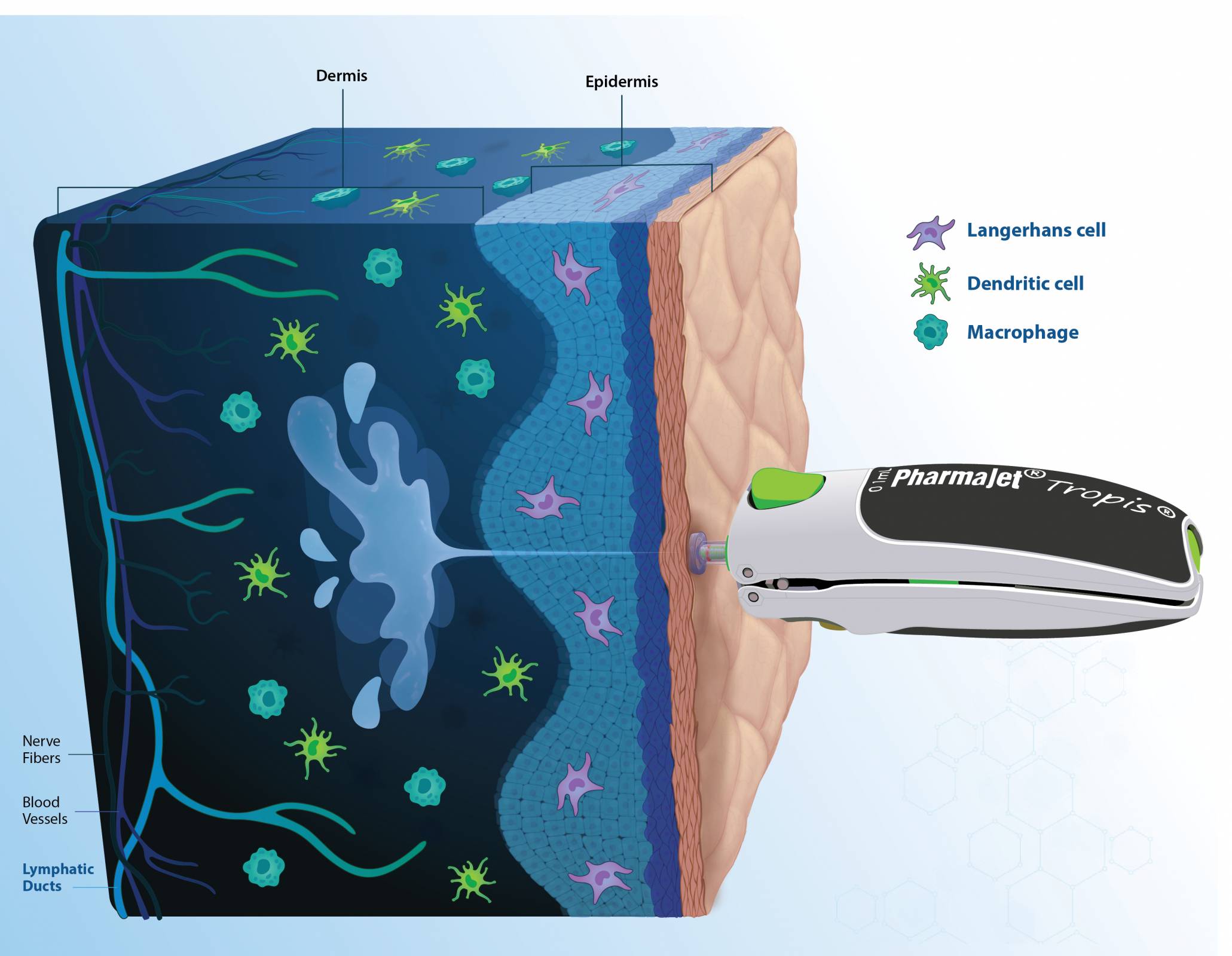
The study's results demonstrated that GEMCOVAC-OM, administered exclusively with Tropis®, is well-tolerated with no related serious adverse events and significantly boosts immune responses against the Omicron variant.
GEMCOVAC®-OM samRNA vaccine low dose was licensed under emergency use authorization in 2023.
This new study is the first time a samRNA vaccine has been developed with a lipid nano-emulsion, and the data show that intradermal administration of this vaccine is safe and well-tolerated.
Furthermore, the April 18, 2024 publication cited that the self-amplifying, thermostable mRNA platform delivered intradermally with Tropis provides a framework for next-generation vaccines that can improve accessibility and global equity.
PharmaJet has partnered with Gennova to improve the performance and outcomes of their samRNA platform with PharmaJet's breakthrough delivery technology.
The PharmaJet pioneering technology unlocks the rich potential of the human dermis, paving the way for enhanced immune responses.
Chris Cappello, President and Chief Executive Officer, PharmaJet, commented in a press release on April 24, 2024, "This new data adds to the evidence base indicating Tropis needle-free ID administration is an enabler for vaccine platforms."
Tropis intradermal administration and Stratis® SC/IM (for intramuscular and subcutaneous administration) are the only commercially scaled needle-free technologies that enhance the performance of several vaccines and therapeutics.
Since its launch, Tropis has performed over 10 million vaccinations in several countries.
Moderna, Inc. and OpenAI today announced their ongoing collaboration to co-innovate with a shared vision of AI's transformative potential in the future of business and healthcare.
Moderna is a digital-first company that has leveraged the power of machine learning since its beginnings.
This strong data foundation and its robust learning culture position the Company to responsibly and seamlessly integrate generative AI into its operations and capitalize on next-generation AI innovation.
The organizations began their collaboration in early 2023 by launching Moderna's instance of ChatGPT, called mChat, internally built on top of OpenAI's API.
With more than 80% internal adoption since its debut, this initial success accelerated an AI culture that led to the deployment of ChatGPT Enterprise and its enhanced capabilities such as Advanced Analytics, Image Generation and GPTs.
These GPTs are now embedded across Moderna's business functions - from legal, to research, to manufacturing, to commercial - and are purpose-built as assistants that work beside Moderna's employees, augmenting their roles through personalized support.
With these tools serving as an extension to Moderna's team, the Company can advance its mission to deliver the greatest possible impact to people through mRNA medicines.
Stéphane Bancel, Chief Executive Officer of Moderna, commented in a press release on April 24, 2024, "Moderna has an ambitious plan to launch multiple products over the next few years, and collaborations with companies like OpenAI are critical to our ability to scale and maximize our impact on patients."

CureVac N.V. today announced the start of the Phase 1 part of a combined Phase 1/2 clinical trial of an investigational influenza A (H5N1) pre-pandemic vaccine candidate developed in collaboration with GSK.
In the initial Phase 1 dose-escalation part of the study, up to five dose levels will be assessed compared to a placebo control. The study will be conducted in the United States.
The monovalent vaccine candidate is based on CureVac's proprietary second-generation messenger ribonucleic acid (mRNA) backbone and encodes an influenza A H5-antigen.
Dr. Myriam Mendila, CureVac's Chief Development Officer, commented in a press release on April 24, 2024, "This clinical milestone, in collaboration with GSK, expands the application of our mRNA technology into an additional indication in infectious diseases and addresses the need to be prepared for potential future pandemics."
The H5N1 avian influenza virus is considered a potential pandemic threat. It is known to sporadically cross-species from its original bird host to other animal hosts, such as bears, cows, foxes, and humans worldwide.
The combined Phase 1/2 study will evaluate the safety, reactogenicity, and immunogenicity of an investigational influenza A (H5N1) pre-pandemic vaccine candidate in healthy younger and older adults.
The broad CureVac-GSK infectious disease collaboration was first announced in July 2020.
As of April 2024, the U.S. government has approved a bird flu vaccine (Audenz) and invested hundreds of millions in preparing avian influenza vaccine candidates.

Since the first dengue-like epidemic was suspected in 1635 in the Caribbean Islands, this mosquito-transmitted disease has impacted the health of millions of people throughout the Region of the Americas.
According to new data, the year 2024 may set an all-time record.
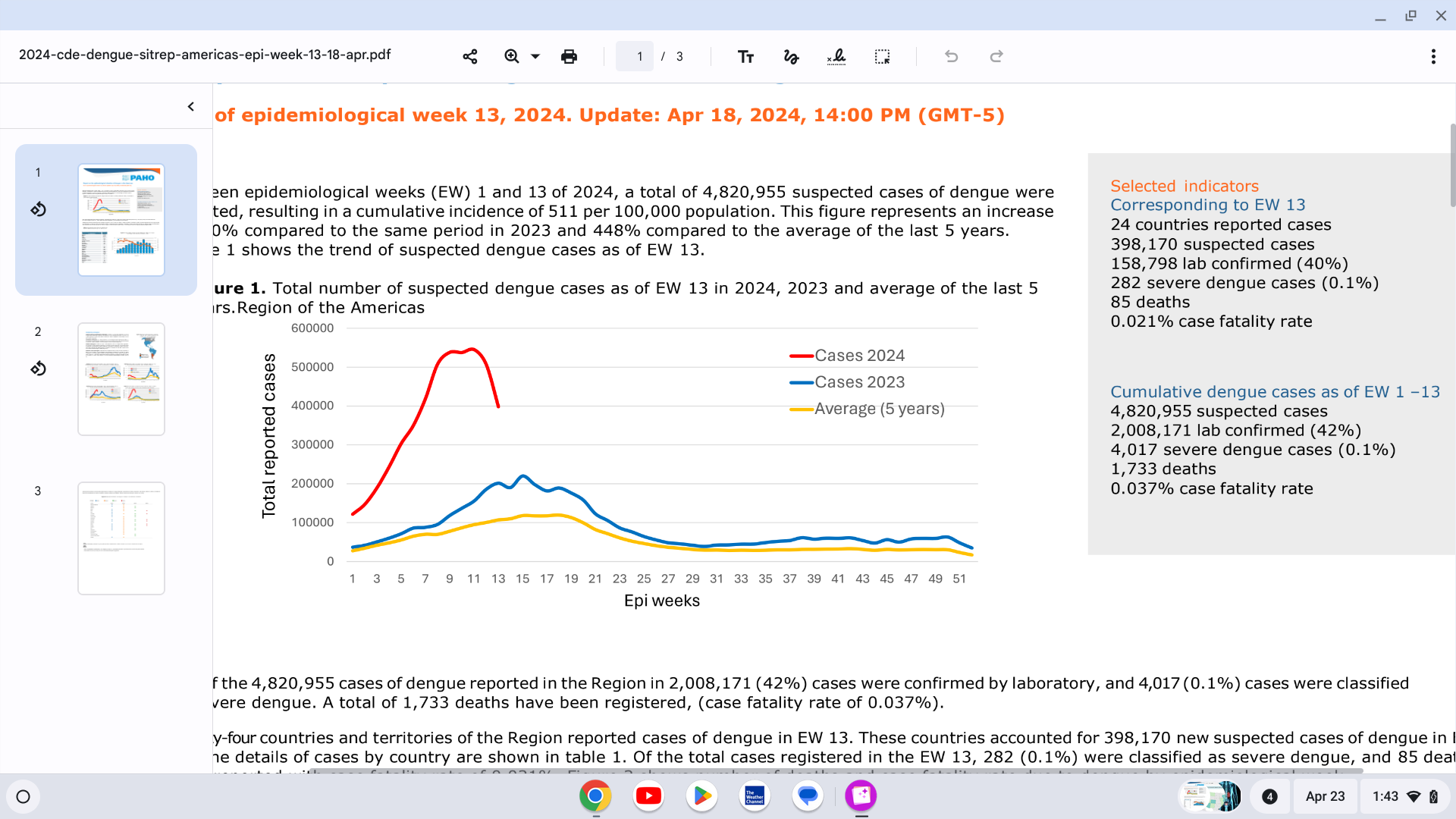
The Pan American Health Organization (PAHO) recently issued Situation Report No. 14 confirming that 4,820,955 suspected cases of Dengue (an increase
of 260% from 2023) and 1,733 deaths have been registered as of mid-April 2024.
As of April 23, 2024, Brazil has reported the most Dengue cases this year.
To alert international travelers, the U.S. Centers for Disease Control and Prevention (CDC) reissued its Level 1 Travel Health Advisory for the Americas on April 18, 2024. The countries listed have reported higher-than-usual dengue cases.
The CDC says travelers to these Caribbean, Central, and South American countries may be at increased risk.
Within the U.S., the CDC reported that 37 jurisdictions have reported 929 dengue cases as of April 2024.
The unfortunate leader is Puerto Rico, which has 644 dengue cases, followed by the state of Florida, which has 106 travel-associated cases and five locally acquired dengue cases this year.
From a prevention perspective, two dengue vaccines have been approved. To learn about vaccination options, the CDC says travelers should speak with a vaccine advisor at least one month before traveling abroad.
As of April 23, 2024, travel vaccines are offered at specialty clinics and certified pharmacies in the U.S.
ImmunityBio, Inc. today announced that the U.S. Food and Drug Administration (FDA) had approved ANKTIVA® (N-803) plus Bacillus Calmette-Guérin (BCG) vaccine for the treatment of patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.
This treatment is essential as bladder cancer is the 10th most commonly diagnosed cancer globally, and the American Cancer Society estimates there will be 83,190 new cases and 16,840 deaths from bladder cancer in the U.S. in 2024.
"The FDA's approval of ANKTIVA marks our launch of a next-generation immunotherapy beyond checkpoint inhibitors," said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on April 22, 2024.
"ANKTIVA not only proliferates and activates the patient's own NK cells and CD8+ killer T cells, but also activates CD4+ T helper cells, thus enhancing the proliferation of memory killer T cells.
ANKTIVA is a novel IL-15 superagonist complex consisting of an IL-15 mutant (IL-15N72D) fused with an IL-15 receptor alpha, which binds with high affinity to IL-15 receptors on NK, CD4, and CD8 T cells.
ANKTIVA is expected to be available in the United States by mid-May 2024, as well as ImmunityBio's Patient Assistance Program.
Merck's TICE® BCG vaccine is used in this therapy.

Moderna, Inc. today announced a contract with Brazil's Ministry of Health (Ministério da Saúde) to supply its monovalent mRNA COVID-19 vaccine.
Under the new contract, 12.5 million doses of Moderna's mRNA COVID-19 vaccine (SpikeVax) are anticipated for delivery in the second quarter of 2024.
This contract follows the Brazilian Health Regulatory Agency's approval of Moderna's COVID-19 vaccine (XBB.1.5 sublineage) in March 2024 to prevent COVID-19 in children from six months of age and adults.
"We are proud to partner with the Ministry of Health to provide our mRNA COVID-19 vaccine for the first time in Brazil as part of the national vaccination campaign," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release on April 22, 2024.
"This agreement underscores our commitment to global health and our role in supporting Brazil's efforts to protect its citizens against COVID-19."
"We look forward to working with the Ministry of Health to help maintain health security in Brazil."
Previously, Moderna generated preclinical and clinical data of its monovalent XBB.1.5 vaccine candidate, which showed an immune response against XBB sublineages and currently circulating strains of the SARS-CoV-2 virus, including JN.1.

Although antiretroviral treatment (ART) can help manage the impact of the Human Immunodeficiency Virus (HIV), it is not a cure. People living with HIV need to take the treatment for their entire lives to suppress viral replication and protect their immune systems.
To address this clinical need, ACTG, a global clinical trials network focused on HIV and other infectious diseases, today announced the opening of A5374, a phase 1/2a study evaluating the safety, tolerability, and antiviral effect of a novel combination regimen that includes therapeutic T-cell vaccines, two broadly neutralizing antibodies (3BNC117-LS, 10-1074-LS), and an immune-system boosting treatment among people living with HIV who started ART during acute HIV infection.
This study hypothesizes that this combination regimen will be safe and result in a more extended period of viral control during a closely monitored pause in ART (known as an analytic treatment interruption) compared to placebo.
The approach being studied in A5374 is part of a potential path toward enabling the immune system to manage HIV in the absence of ART for weeks or months.
“We expect that controlling HIV in the absence of ART will require a combination of novel treatments to decrease the number of cells harboring HIV and simultaneously stimulate the immune system to keep the virus from replicating,” said ACTG Chair Judith Currier, M.D., M.Sc., University of California Los Angeles in a press release.
“A5374 is a pivotal trial, and we hope it will provide us with important insights into how to boost the immune system to control HIV.”
ACTG says carefully monitored analytic treatment interruptions are an essential part of HIV cure clinical trials. They can help determine whether a research intervention can reduce latent HIV (HIV that is “hidden” in different cells and tissues but not replicating) or increase immune function and delay or prevent latent HIV from actively replicating.
While there are no U.S. FDA-approved HIV prevention vaccines today, clinical development accelerated in 2023, with vaccine candidates utilizing innovative technologies such as mRNA.