Search API
Once the Zika fever outbreak subsided in 2015, many public health agencies refocused on chikungunya and dengue outbreaks.
However, continued Zika outbreaks in the Region of the Americas have demonstrated how a relatively obscure mosquito-borne disease can become a persistent health risk.
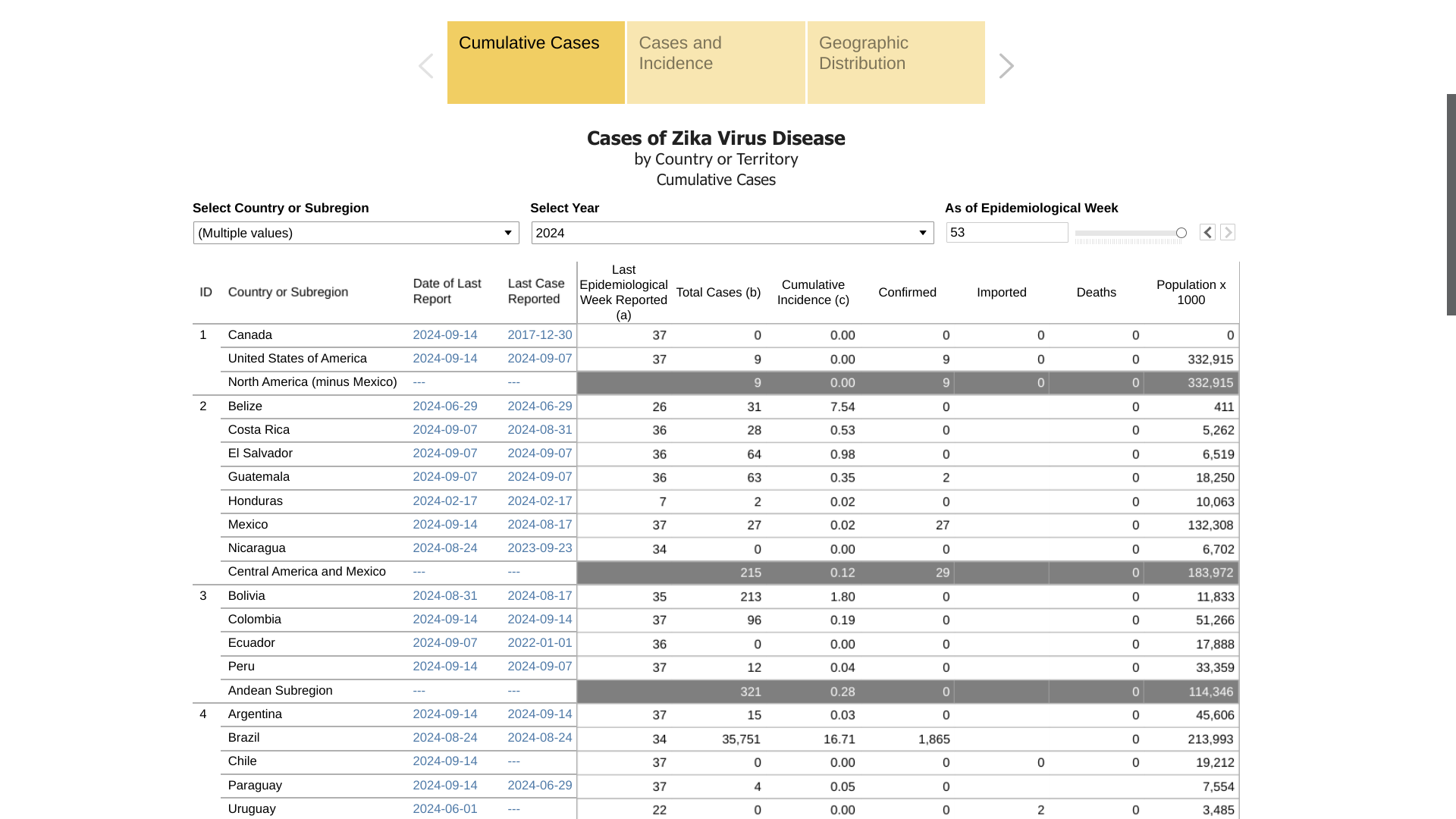
As of September 24, 2024, the Pan American Health Organization (PAHO) reported 36,331 Zika cases this year.
While most people infected with Zika will recover, many infants have been severely impacted by microcephaly. Researchers revealed over 200 microcephaly cases in Brazil.
The PAHO says pregnant women should avoid visiting Zika-endemic countries such as Brazil.
In the United States, the San Juan, Puerto Rico Department of Health says Zika will continue to infect people. As of September 2024, 16 Zika cases have been reported in Puerto Rico.
As of 2024, no approved Zika vaccines exist, but vaccine candidates are conducting clinical research. Valneva SE's second-generation VLA1601 vaccine launched a phase 1 study, with results expected in 2025.
Cidara Therapeutics, Inc., today announced the first subjects dosed in the Phase 2b NAVIGATE trial in the U.S. and U.K. The trial evaluates the efficacy and safety of CD388, a long-acting antiviral, for the pre-exposure prophylaxis of influenza during the 2024-2025 flu season.
CD388 is an investigational drug-Fc conjugate (DFC) comprised of multiple copies of a potent small molecule neuraminidase inhibitor stably conjugated to a proprietary Fc fragment of a human antibody.
DFCs are not vaccines or monoclonal antibodies but low-molecular-weight biologics designed to function as long-acting small-molecule inhibitors.
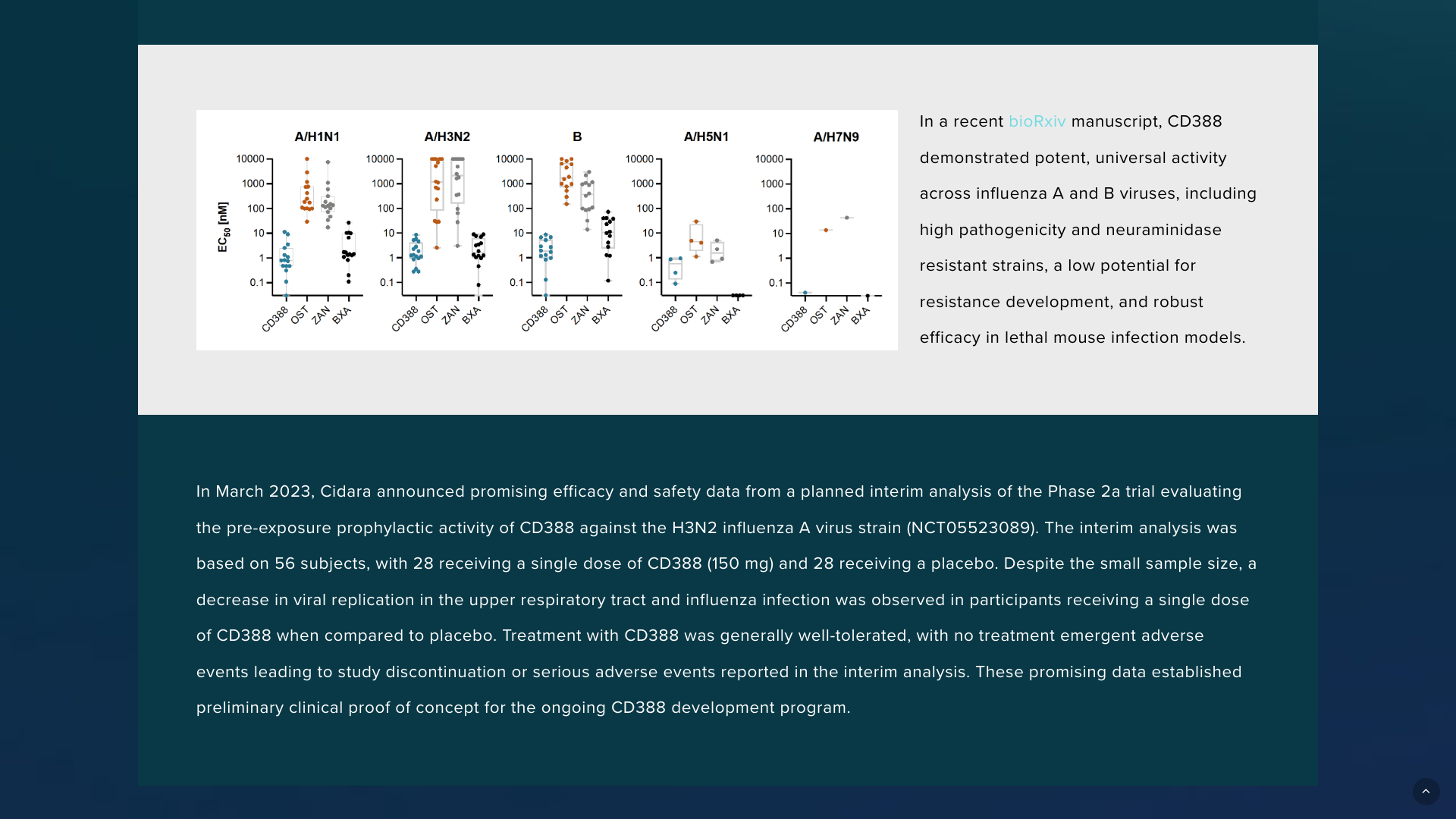
In a June 2024 bioRxiv manuscript, CD388 demonstrated potent, universal activity across influenza A and B viruses, including high pathogenicity and neuraminidase-resistant strains, a low potential for resistance development, and robust efficacy in lethal mouse infection models.
“Effective new options are needed to prevent influenza, particularly for people who do not respond well to seasonal flu vaccines,” said Jeffrey Stein, Ph.D., president and chief executive officer of Cidara Therapeutics, in a press release on September 23, 2024.
“Since CD388 is not a vaccine, its activity does not rely upon an immune response and is, thereby, expected to work regardless of immune status."
"In addition, CD388 has demonstrated the potential to prevent infection by both seasonal and pandemic strains of influenza A and B."
The Phase 2b clinical trial is a randomized, double-blind, controlled trial targeting 5,000 healthy, unvaccinated adult subjects who are not at risk of complications from influenza. Three CD388 dose groups or a placebo will be administered to subjects as a single dose at the beginning of the influenza season. Subjects will then be followed for the remainder of the influenza season to monitor for breakthrough cases.
Cidara Therapeutics is a biotechnology company using its proprietary Cloudbreak® platform to develop DFC immunotherapies designed to save lives and improve the standard of care for patients facing serious diseases.
The U.S. Centers for Disease Control and Prevention (CDC) today issued Health Alert Network Health Update (CDCHAN-00516) to provide additional information about the ongoing outbreak of clade I monkeypox virus (MPXV), the virus that causes mpox.
As of September 23, 2024, no cases of clade I mpox have been identified in the U.S.
This mpox strain is more severe than the clade 2 strain circulating in the U.S. since May 2022.
The CDC urges travelers to vaccinate against mpox if they are heading to Eastern and Central African countries where clade 1 MPXV has been spreading.
Furthermore, healthcare providers and travel vaccine experts should recommend vaccination to those whose activities place them at risk.
This is essential advice since clade 1 mpox cases have recently been confirmed in international travelers.
For example, The Mint reported a man from Malappuram district in Kerala, India, has been detected with Mpox clad I. The patient had returned from the United Arab Emirates.
According to the CDC, two doses of JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) should be given at least six weeks before traveling abroad. This U.S. FDA-approved vaccine is available at clinics and pharmacies in the U.S.

A new report jointly released by the World Health Organization (WHO) and ITU (International Telecommunication Union) today suggests an additional $0.24 per patient per year investment in digital health interventions, such as telemedicine, mobile messaging, and chatbots, can help save more than two million lives over the next decade from noncommunicable diseases.
These diseases include, but are not limited to, cardiovascular, cancer, diabetes, and chronic respiratory diseases.
This small investment could also avert approximately 7 million acute events and hospitalizations, significantly reducing the strain on healthcare systems worldwide.
ITU Secretary-General Doreen Bogdan-Martin commented in a press release on September 23, 2024, "We call for greater collaboration between the health and tech sectors, including the development of strong digital public infrastructure, essential for the delivery of digital health services that can benefit people everywhere without leaving anyone behind."
The publication" "going Digital for Noncommunicable Diseases: The Case for Action" was launched at an event hosted by the Government of The Gambia during the 79th United Nations General Assembly in collaboration with the ITU and WHO.

Vicebio Ltd. today announced a $100 million Series B financing led by TCGX and others. Vicebio is developing next-generation vaccines for respiratory viruses utilizing its proprietary Molecular Clamp technology, which was discovered at the University of Queensland (UQ).
The Molecular Clamp technology applies to a wide range of viruses, including Respiratory Syncytial Virus (RSV), Human Metapneumovirus (hMPV), Parainfluenza virus, Influenza, and Coronaviruses, as confirmed by promising preclinical and clinical studies.
Vicebio has recently initiated a Phase I clinical trial with VXB-241, its bivalent vaccine targeting RSV and hMPV. Initial clinical readouts of the Phase 1 study are expected to launch in mid-2025.
This financing will also support the acceleration and expansion further development of Vicebio’s multivalent pipeline, including VXB-251, a trivalent vaccine targeting RSV, hMPV, and Parainfluenza Virus 3, a further valency that addresses a significant remaining medical burden in the elderly.
Cariad Chester, Managing Partner at TCGX, said in a September 23, 2024, press release, “Vicebio has a unique capability to advance vaccine products that simultaneously provide robust immune responses against multiple respiratory pathogens. We look forward to working closely with the team to bring these important vaccines to the market.”
Prof. Paul Young, Daniel Watterson, and Keith Chappell at UQ developed the Molecular Clamp proprietary technology.

The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended marketing authorization for the companies’ Omicron KP.2-adapted monovalent COVID-19 vaccine (COMIRNATY ® KP.2) for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals six months of age and older.
Pfizer Inc. and BioNTech SE announced that the European Commission will review the CHMP’s recommendation and is expected to make a final decision soon.
The CHMP recommendation dated September 19, 2024, is based on the non-clinical and manufacturing data of the Omicron KP.2-adapted vaccine and the clinical and real-world evidence supporting the safety and efficacy of prior formulas of the COVID-19 vaccines by Pfizer and BioNTech.
Pending the EC's authorization of the Omicron KP.2-adapted vaccine, both the Omicron KP.2-adapted vaccine and the Omicron JN.1-adapted vaccine will be available across the EU, though availability will vary based on individual country government requests and national recommendations.

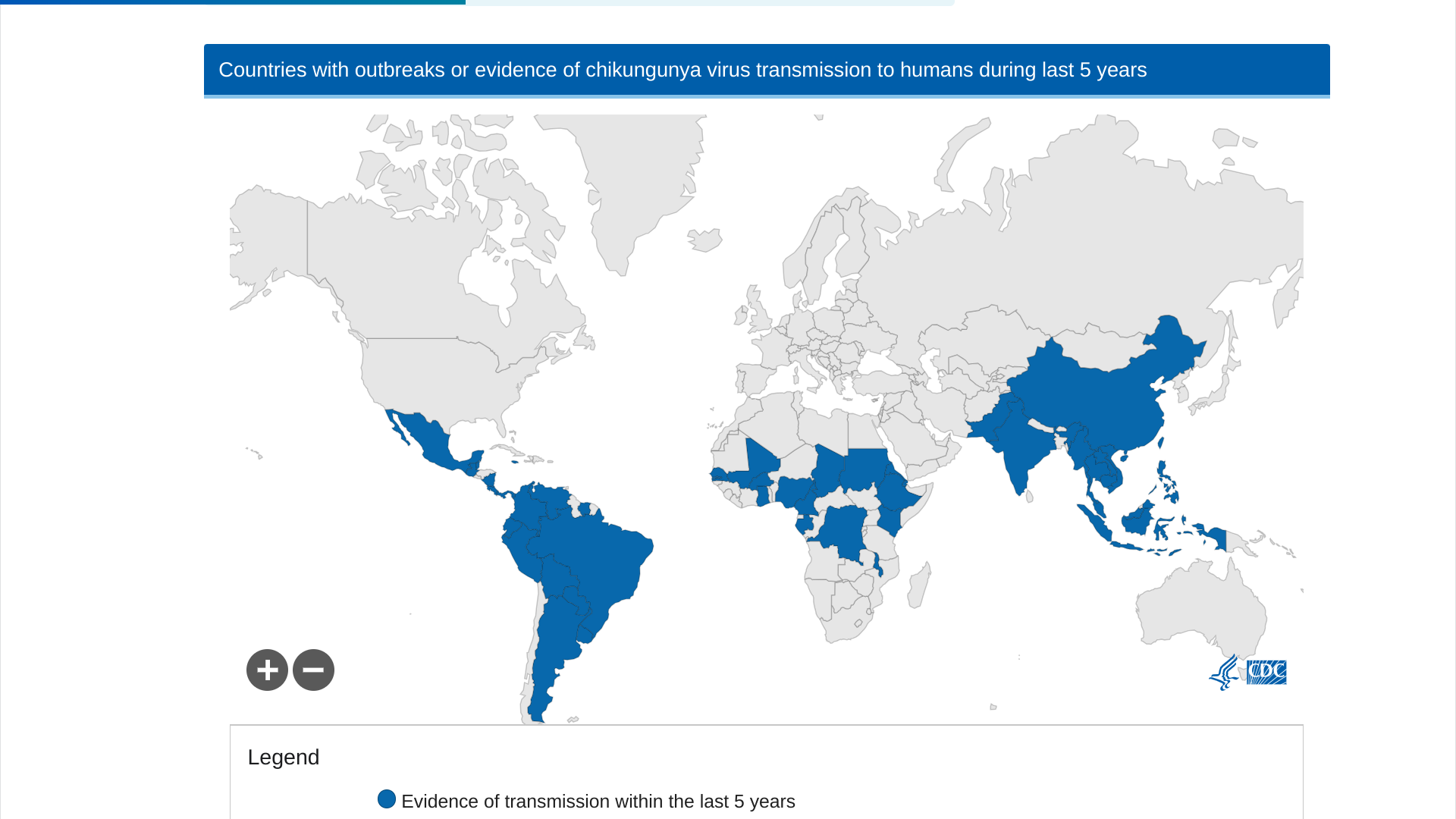
The peer-reviewed journal The Lancet Infectious Diseases recently published interim results of a double-blind, randomized, placebo-controlled phase 3 trial in adolescents of the U.S. FDA-approved, single-dose IXCHIQ® (VLA1553) chikungunya vaccine.
In an article published on September 4, 2024, these researchers concluded that VLA1553 was generally safe and induced seroprotective titers in almost all vaccinated adolescents, with favorable safety data in seropositive adolescents at baseline.
VLA1553 induced seroprotective chikungunya virus neutralizing antibody levels in 247 of 250 (98.8%, 95% CI 96·5–99·8) participants 28 days after vaccination.
This data supports using VLA1553 to prevent diseases caused by the chikungunya virus among adolescents and in endemic areas in the Region of the Americas.
As of September 22, 2024, the Pan American Health Organization reported 390,669 CHIKV cases. Specifically, Brazil has confirmed 170 related deaths this year.
If you are traveling to an area at risk for chikungunya, the U.S. CDC suggests discussing vaccination options with your healthcare provider at least one month before departing abroad.