Search API
Pfizer Inc. recently announced positive top-line data from the ongoing pivotal Phase 3 clinical trial evaluating a single dose of ABRYSVO versus placebo in adults 18 to 59 years of age at risk of developing severe respiratory syncytial virus (RSV)-associated lower respiratory tract disease (LRTD).
Highlights from this study include participants' achieving at least a fourfold increase in serum neutralizing titers for RSV-A and RSV-B one month following receipt of ABRYSVO compared to pre-vaccination.
During the trial, ABRYSVO was well-tolerated, and safety findings were consistent with those from previous investigations of ABRYSVO in other populations.
Among these U.S. adults, 9.5% have a chronic condition that puts them at risk of severe RSV disease, and this percentage rises to 24.3% among persons 50 to 64 years of age.
However, no RSV vaccines have been approved for adults aged 18 to 59.
Annaliesa Anderson, Ph.D., Senior Vice President and Head, Vaccine Research and Development, Pfizer, stated in a press release on April 9, 2024, "We are excited to address a significant unmet need, pending regulatory authority approval, as ABRYSVO has the potential to become the first and only RSV vaccine for adults 18 years and older."
In May 2023, the FDA approved ABRYSVO for the prevention of LRTD caused by RSV in individuals 60 years of age or older.
Pfizer is currently the only company that has an approved RSV vaccine for protecting older adults and infants through maternal immunization.
The respiratory syncytial virus causes RSV disease. There are two major subgroups of RSV: RSV-A and RSV-B. Both subgroups cause disease and can co-circulate or alternate predominance from season to season.

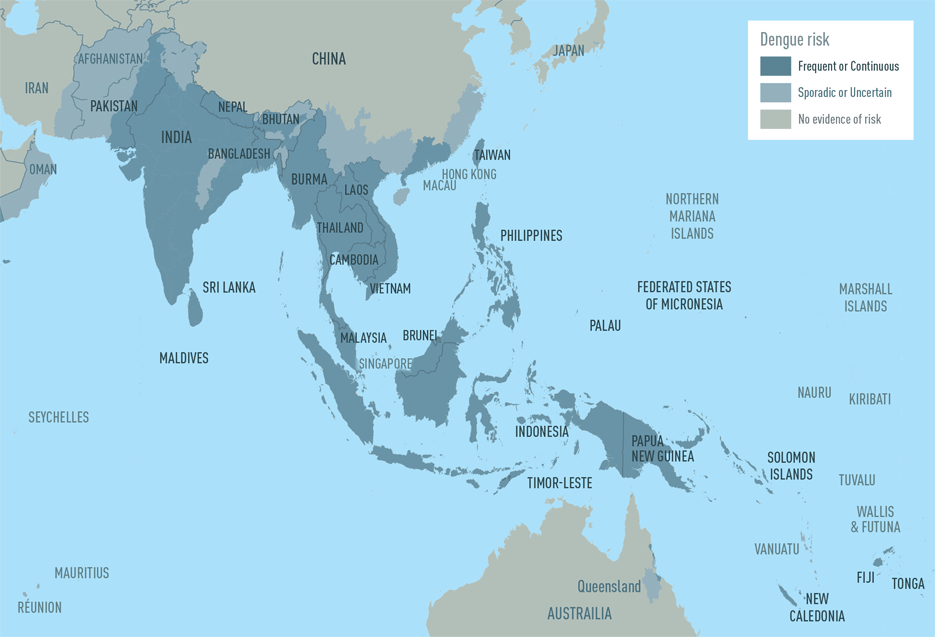
While dengue virus outbreaks in the Caribbean have received a lot of attention, travelers visiting the Pacific Islands and Asia should also be vigilant.
According to the U.S. Centers for Disease Control and Prevention (CDC), countries such as Cambodia, Indonesia, Laos, Malaysia, Singapore, and Sri Lanka are reporting dengue outbreaks in 2024.
For example, Singapore's National Environment Agency reported over 5,000 dengue cases in the first quarter of 2024, more than double the 2,360 cases reported in the same period in 2023.
On April 18, 2024, the CDC reissued a Level 1—Practice Usual Precautions, Travel Health Advisory for the western Pacific area. Dengue can become severe within a few hours and is a medical emergency that usually requires hospitalization.
Because infected mosquito bites spread dengue, all travelers to high-risk areas should prevent mosquito bites by using an EPA-registered insect repellent, wearing long-sleeved shirts and long pants when outdoors, and sleeping in an air-conditioned room or room with window screens or under an insecticide-treated bed net, says the CDC.
Furthermore, adults traveling to the areas should speak with a travel vaccine advisor about one month before departure regarding vaccination options.
As of April 25, 2024, there are two approved dengue vaccines and several conducting late-stage clinical research.
In an essential step forward for malaria prevention in Africa, Benin, Liberia, and Sierra Leone today announced the launching of large-scale malaria vaccinations targeting millions of children across the three West African nations.
Today's launch confirms eight countries on the African continent have the opportunity to offer malaria vaccinations.
As of April 25, 2024, more than 30 countries in the African region are scheduled to roll out malaria vaccinations over the next year through support from Gavi, the Vaccine Alliance.
The World Health Organization (WHO) has recommended Mosquirix™ (RTS,S/AS01) and R21 / Matrix-M™ malaria vaccines.
A pilot malaria vaccine program in Ghana, Kenya, and Malawi reached over two million children from 2019 to 2023, showing a significant reduction in malaria illness, a 13% drop in overall child mortality, and substantial reductions in hospitalizations.
Aurelia Nguyen, Chief Programme Officer at Gavi, the Vaccine Alliance, commented in a press release, "Today we celebrate more children gaining access to a new lifesaving tool to fight one of Africa's deadliest diseases."
"Introducing malaria vaccines into routine programs in Benin, Liberia, and Sierra Leone alongside other proven interventions will help save lives and offer relief to families, communities, and hard-pressed health systems."
As of April 2024, neither malaria vaccine is available in the United States.
Malaria outbreaks remain a considerable health challenge in the African region, which is home to 11 countries that carry approximately 70% of the global burden of malaria.
According to the World Malaria Report 2023, the region accounted for 94% of global malaria cases and 95% of all malaria deaths in 2022.
In the U.S., about 2,000 travel-related malaria cases are reported annually. In the State of Florida, travel-related and locally acquired malaria cases have been reported over the past year.
To receive local travel vaccine recommendations, PassportHealth Tampa offers pre-departure advice.
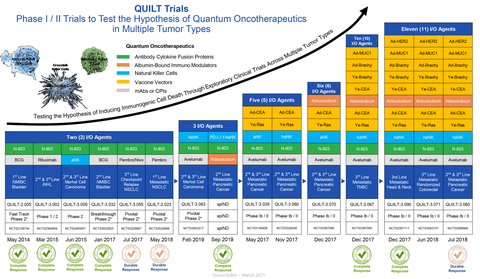
ImmunityBio, Inc. today announced positive overall survival results in the QUILT 3.055 study of 2nd- and 3rd-line Non-small cell lung cancer (NSCLC) patients who progressed after checkpoint inhibitor therapy and standard-of-care chemotherapy.
The clinical study's results continue to reinforce ImmunityBio's belief in the unique mechanism of action of ANKTIVA® (N-803) and its potential efficacy as a next-generation immunotherapy across multiple solid and liquid tumor types.
The positive overall survival data of patients enrolled in QUILT 3.055, a basket trial across multiple tumor types in which checkpoint inhibitors failed, will be discussed, along with the status of launch readiness for ANKTIVA for its recently approved indication in Non-Muscle-Invasive Bladder Cancer. (NMIBC) on an investor conference call on April 26 at 8 am PDT.
"The results we noted with the completion of the QUILT 3.055 basket trial across multiple tumor types in patients with late-stage cancers for whom the standard of care plus checkpoints failed validates our hypothesis that orchestration of NK cells with killer T cells and memory T cells could result in meaningful clinical improvements to current standards of care."
"We hypothesized that activation and proliferation of natural killer cells through IL-15 stimulation could rescue T cells after checkpoint failure, regardless of tumor type or location. As with non-muscle invasive bladder cancer, we believe that ANKTIVA enhanced the NK and T cell activity critical for targeting and killing cancer cells which have entered the phase of tumor evasion and resistance," said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on April 25, 2024.
"The findings of a significant extension of overall survival in 2nd—and 3rd-line lung cancer affirm that combination therapy, with the orchestration of the innate and adaptive immune system, could potentially lead to the evolution of immunotherapy beyond T cells for all cancer patients."
"We are committed to pursuing additional indications for ANKTIVA in our pipeline with a mission to deliver new hope to patients with serious, advanced cancers where standard therapies have failed."
In NSCLC patients who relapsed or were refractory to checkpoint inhibitors, ANKTIVA was administered together with the same checkpoint inhibitor.
The addition of ANKTIVA resulted in the rescue of the checkpoint therapy efficacy, with significant prolongation of overall survival.
These positive results were noted regardless of the patient's PD-L1 status, consistent with the mechanism of action of ANKTIVA in activating and proliferating natural killer cells and stimulating CD8+ Killer Memory T cells.
This prolongation of survival in NSCLC following checkpoint failure is consistent with ImmunityBio's findings of durable, complete responses following BCG failure in NMIBC.
A meeting with the U.S. FDA has been scheduled for June 2024 to discuss the company's overall survival results in PD-L1 negative and positive patients and registration plans for 2nd-line and 3rd-line NSCLC patients whose cancer did not respond or continue to respond to checkpoint therapy and for whom few alternative therapies are available.
Novavax Inc. recently announced its Nuvaxovid XBB.1.5 dispersion for injection, COVID-19 Vaccine (recombinant, adjuvanted) (NVX-CoV2601) became available to pharmacies across the United Kingdom (U.K.) for private healthcare providers to offer an updated protein-based non-mRNA option as part of the 2024 vaccination season.
On April 18, 2024, Novavax stated in a press release, Today is an important milestone for our organization. It demonstrates our commitment to deliver an updated protein-based COVID-19 vaccine as we evolve into an endemic market.
It also underscores our place as a long-term partner to public health.
We believe a diverse vaccine portfolio and broader access can be vital in protecting communities in the U.K. and elsewhere.
Our participation in the U.K. private market follows the receipt of a Wholesale Dealers License from the U.K. Medicines and Healthcare Products Regulatory Agency and the U.K. Health Security Agency’s latest Green Book update in February 2024 includes our updated COVID-19 vaccine for active immunization to prevent COVID-19 in individuals aged 12 and older.

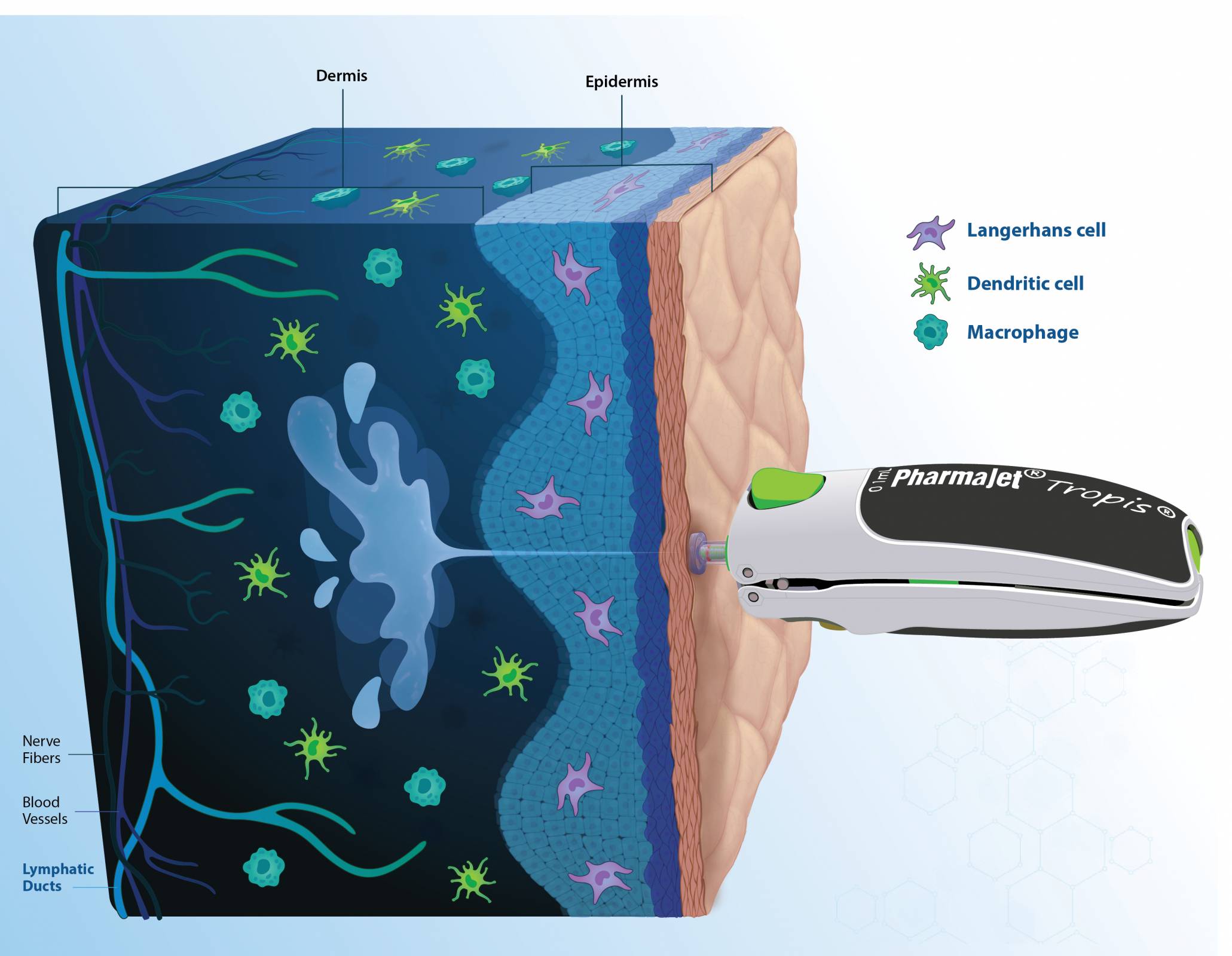
PharmaJet® today announced that the journal Nature published results from Gennova Biopharmaceutical's Phase 2/3 clinical trial. The trial was conducted to evaluate the safety and immunogenicity of its novel samRNA-based Covid-19 vaccine booster.
The study's results demonstrated that GEMCOVAC-OM, administered exclusively with Tropis®, is well-tolerated with no related serious adverse events and significantly boosts immune responses against the Omicron variant.
GEMCOVAC®-OM samRNA vaccine low dose was licensed under emergency use authorization in 2023.
This new study is the first time a samRNA vaccine has been developed with a lipid nano-emulsion, and the data show that intradermal administration of this vaccine is safe and well-tolerated.
Furthermore, the April 18, 2024 publication cited that the self-amplifying, thermostable mRNA platform delivered intradermally with Tropis provides a framework for next-generation vaccines that can improve accessibility and global equity.
PharmaJet has partnered with Gennova to improve the performance and outcomes of their samRNA platform with PharmaJet's breakthrough delivery technology.
The PharmaJet pioneering technology unlocks the rich potential of the human dermis, paving the way for enhanced immune responses.
Chris Cappello, President and Chief Executive Officer, PharmaJet, commented in a press release on April 24, 2024, "This new data adds to the evidence base indicating Tropis needle-free ID administration is an enabler for vaccine platforms."
Tropis intradermal administration and Stratis® SC/IM (for intramuscular and subcutaneous administration) are the only commercially scaled needle-free technologies that enhance the performance of several vaccines and therapeutics.
Since its launch, Tropis has performed over 10 million vaccinations in several countries.