Search API
Bavarian Nordic A/S announced an agreement with UNICEF to supply 500,000 MVA-BN® mpox vaccines for African countries impacted by the mpox clade 1 outbreak.
Under the agreement, UNICEF will procure 1 million doses of the vaccine, including the 500,000 doses recently committed by Gavi. Bavarian Nordics intends to make all doses available for supply before the end of 2024.
In the agreement, UNICEF has negotiated a price of up to $65 per vaccine dose, the lowest price in the market.
UNICEF says, 'The mpox virus does not discriminate. Anyone exposed to the virus can become infected, and children, immunocompromised individuals, and pregnant women are at risk of severe disease.'
Paul Chaplin, President & CEO of Bavarian Nordic, said in a press release on September 26, 2024, “Combined with donations by various governments, institutions, and Bavarian Nordic, this agreement has helped to secure more than 2.5 million doses of MVA-BN, thus fulfilling the short-term requirement as expressed by the Africa CDC."
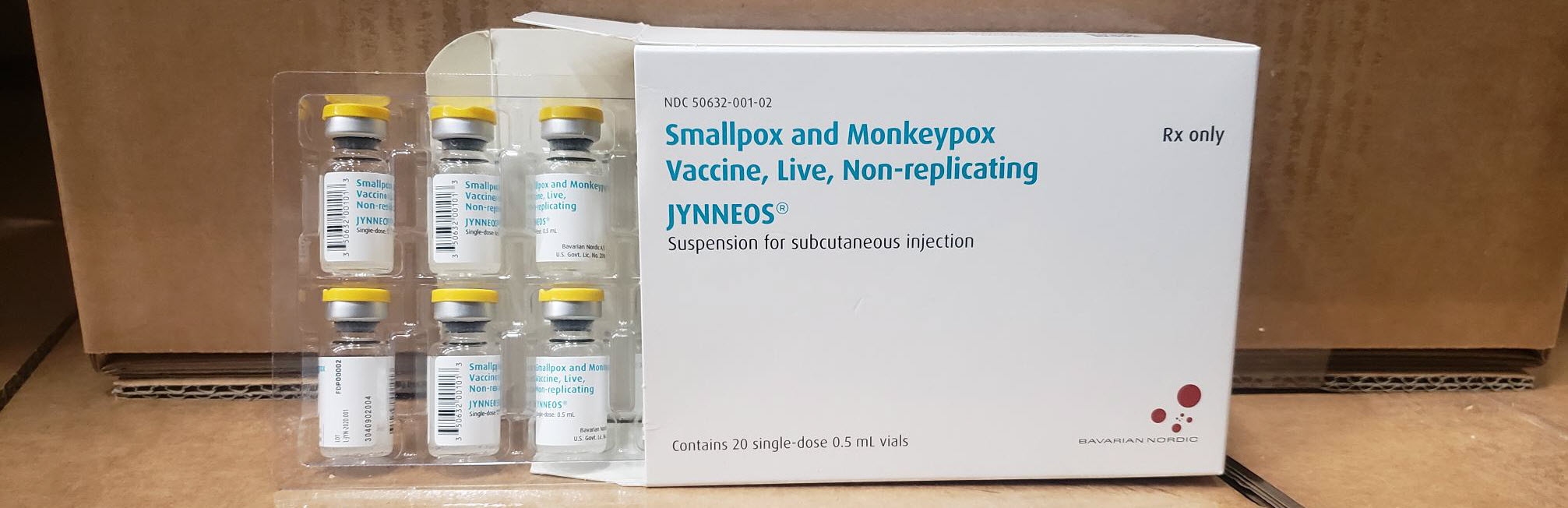
MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic is the only non-replicating mpox - smallpox vaccine approved in the U.S., Switzerland, Singapore, Mexico (marketed as JYNNEOS®), Canada (IMVAMUNE®), and the EU/EAA and United Kingdom (IMVANEX®).
In the U.S., JYNNEOS vaccines are commercially offered at health clinics and pharmacies.
CSL Seqirus announced today that, through its public-private partnership with the U.S. Biomedical Advanced Research and Development Authority (BARDA), the company will expand its Vendor Managed Inventory (VMI) program for its proprietary MF59® adjuvant.
As of September 25, 2024, this is the fifth award CSL Seqirus has received from BARDA in response to sustained highly pathogenic avian influenza (HPAI) activity.
MF59® from the VMI program can be used to manufacture vaccines to protect people against the threat of avian (bird flu) and other strains of influenza. When combined with influenza antigens in a vaccine, MF59® adjuvant is designed to enhance and broaden the body's immune response by creating a broad, cross-reactive antibody response.
Under the terms of the $121.4 million multi-year award, CSL Seqirus will deliver MF59® adjuvant to increase the inventory of the VMI program to 40 million equivalent doses.
"Once again, we're honored to partner with BARDA on pandemic preparedness," said Marc Lacey, CSL Seqirus, Global Executive Director for Pandemic, in a press release.
"This expanded program will increase outbreak resilience and help to protect against threats such as avian influenza."
MF59® will be manufactured at CSL Seqirus' Holly Springs, North Carolina facility.
In April 2024, Dr. Peter Marks, with the U.S. FDA, informed the media that the U.S. stockpile of avian flu-specific vaccines would work well if deployed. As of September 2024, FDA-approved avian influenza vaccines are not commercially available.
Furthermore, the FDA clarified annual flu shots are unlikely to protect people during bird flu pandemics.

When vacationing in Costa Rica this winter, it's best to avoid mountainous areas where mosquitos are transmitting diseases to people.
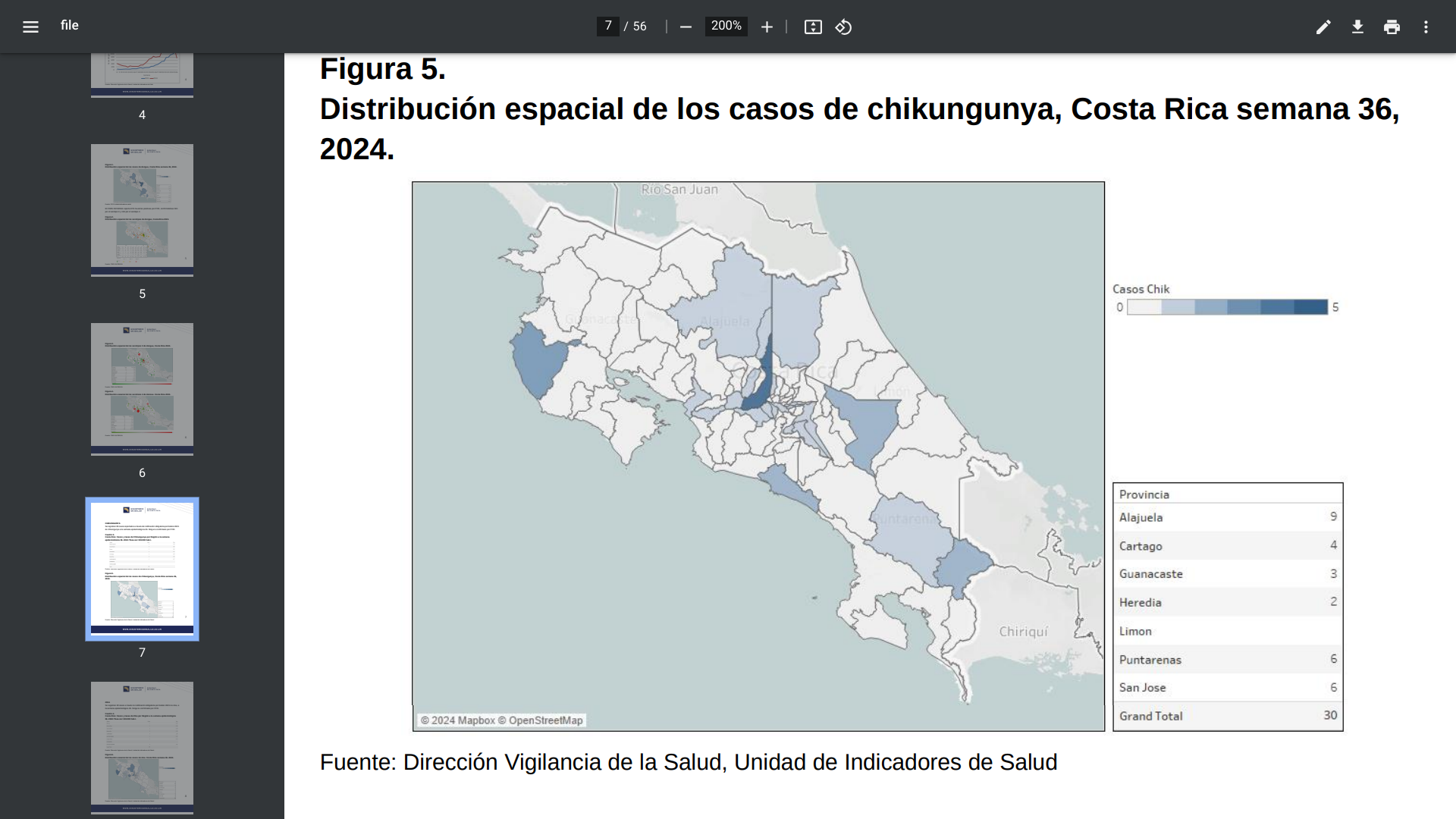
The Costa Rica Health Department's Boletín Epidemiológico N°36 was posted on September 20, 2024. It confirms that mosquito-transmitted Chikungunya, Dengue, Malaria, or Zika virus infections have occurred this year and vary by location.
For example, 30 chikungunya infections have been reported this year, led by the Central Norte, Pacifico Central, and Central Sur regions. Last year, 82 chikungunya cases were confirmed in Costa Rica.
As of 2024, Canada, the United Kingdom, the World Health Organization (PAHO/WHO), and the U.S. CDC have issued travel advisories and vaccine recommendations for Costa Rica.
The CDC recommends checking the recommended vaccine list and seeing a healthcare provider at least a month before visiting Costa Rica. In particular, the CDC now endorses Valneva SE's IXCHIQ® monovalent, single-dose vaccine when visiting Chikungunya endemic areas.
Additionally, the U.S. Embassy in San Jose publishes health topics and recommends visitors enroll in NEWSMART to make it easier to contact you during an emergency while in Costa Rica.
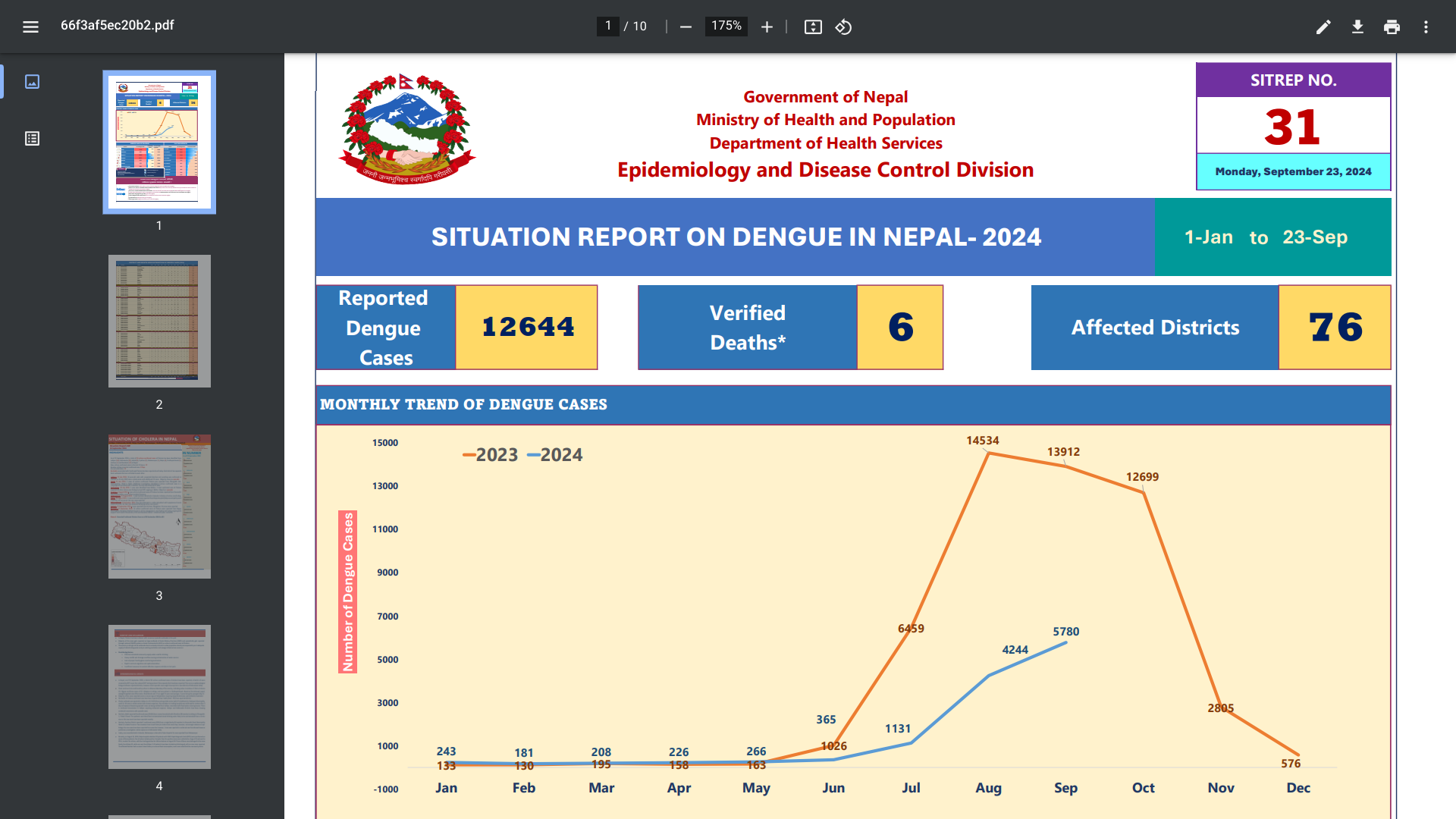
The Federal Democratic Republic of Nepal health department today confirmed over 12,000 cases of dengue and six related deaths in 77 districts this year.
As of mid-September 2024, Nepal's health authorities have abandoned preventive measures such as mosquito search-and-destroy, leading to a rise in infections.
Public health experts warn that dengue outbreaks could escalate in the pending post-monsoon season, which is considered the primary dengue season.
"The coming days will be more challenging for us, as dengue cases could surge," said Dr. Sher Bahadur Pun, chief of the Clinical Research Unit at Shukraraj Tropical and Infectious Disease Hospital at Teku in Kathmandu, as reported by The Kathmandu Post on September 25, 2024.
Nepal reported its first travel-related dengue case in 2004 in the Chitwan district. Since then, Nepal has confirmed an increasing number of outbreaks. In 2023, at least 20 persons died, and the mosquito-transmitted virus infected more than 52,000 people.
Current data suggests that dengue cases are expanding out of the lowland areas typically suitable for breeding Aedes mosquitoes to higher elevations.
Nepal is mainly situated in the Himalayas Mountains, including Mount Everest, at 23,600 feet above sea level. Its experience differs from that of the Region of the Americas, where dengue cases are generally reported below 6,500 feet in elevation.
According to media reporting, Nepal has not launched a dengue vaccination program.
The American Lung Association (ALA) today announced it is launching an educational campaign to help raise awareness about respiratory syncytial virus (RSV) and steps to help prevent RSV infection.
RSV is a highly contagious virus that spreads through close contact with infected individuals.
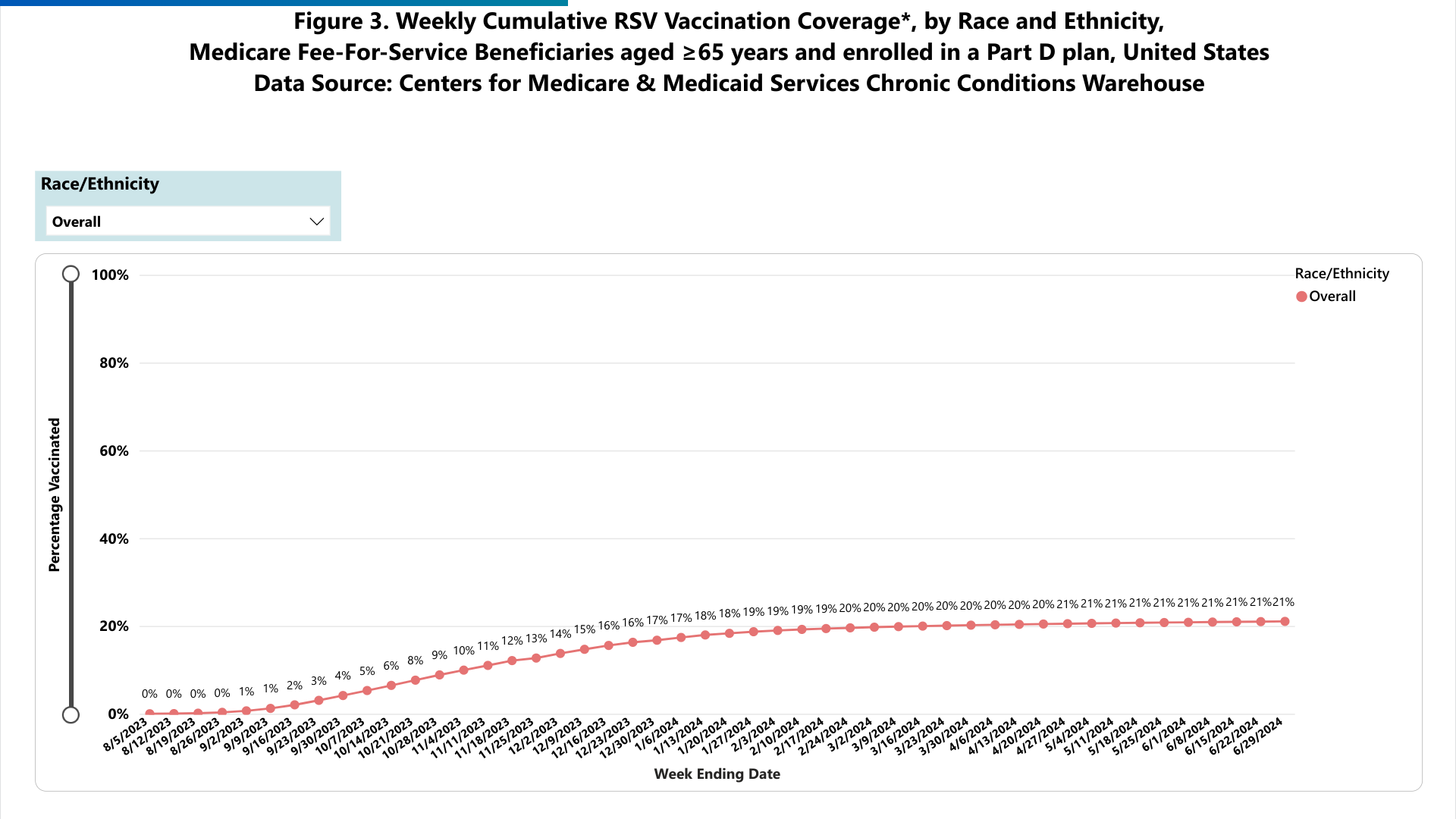
This campaign is essential since only 24% of older adults have received one of the three approved RSV vaccines.
“RSV poses a serious threat, particularly to older adults and those with certain chronic medical conditions. Raising awareness about protection through vaccination is critical to saving lives and reducing hospitalizations during the respiratory virus season,” said Harold Wimmer, President and CEO of the ALA, in a press release on September 25, 2024.
“We encourage individuals 60 and older to talk to their healthcare providers about whether RSV vaccination is recommended for them.”
Individuals who have already received an RSV vaccine do not need additional doses, and vaccination is not recommended annually.
The U.S. Centers for Disease Control and Prevention (CDC) recommends RSV vaccination for adults 75 years and older and for adults aged 60 to 74 who are at high risk for severe disease.
The CDC says the ideal time for vaccination is before the RSV virus spreads widely. It typically increases during the fall and peaks in winter.
The CDC recently reported that RSV levels remained low but were increasing. As of September 2024, the state of Florida had reported the most RSV cases in the United States.
Pfizer Canada ULC and BioNTech SE announced today that Health Canada has authorized the KP.2 variant-adapted COMIRNATY® COVID-19 vaccine for people six months and older.
As of September 24, 2024, the newly formulated vaccine will be available nationwide in pharmacies and vaccination centers in the fall of 2024. Each province and territory will have its pathway for accessing the vaccine, and individuals are encouraged to refer to their provincial/territorial authorities for more information.
The adapted COVID-19 vaccine will be available in Canada as a single dose for individuals five years and older, regardless of prior COVID-19 vaccination history.
For children six months through 4 years of age, COMIRNATY® is authorized for administration as a three-dose series in those without a history of completion of a COVID-19 primary vaccination course or as a single dose for those with a history of completion of a COVID-19 primary vaccination course.
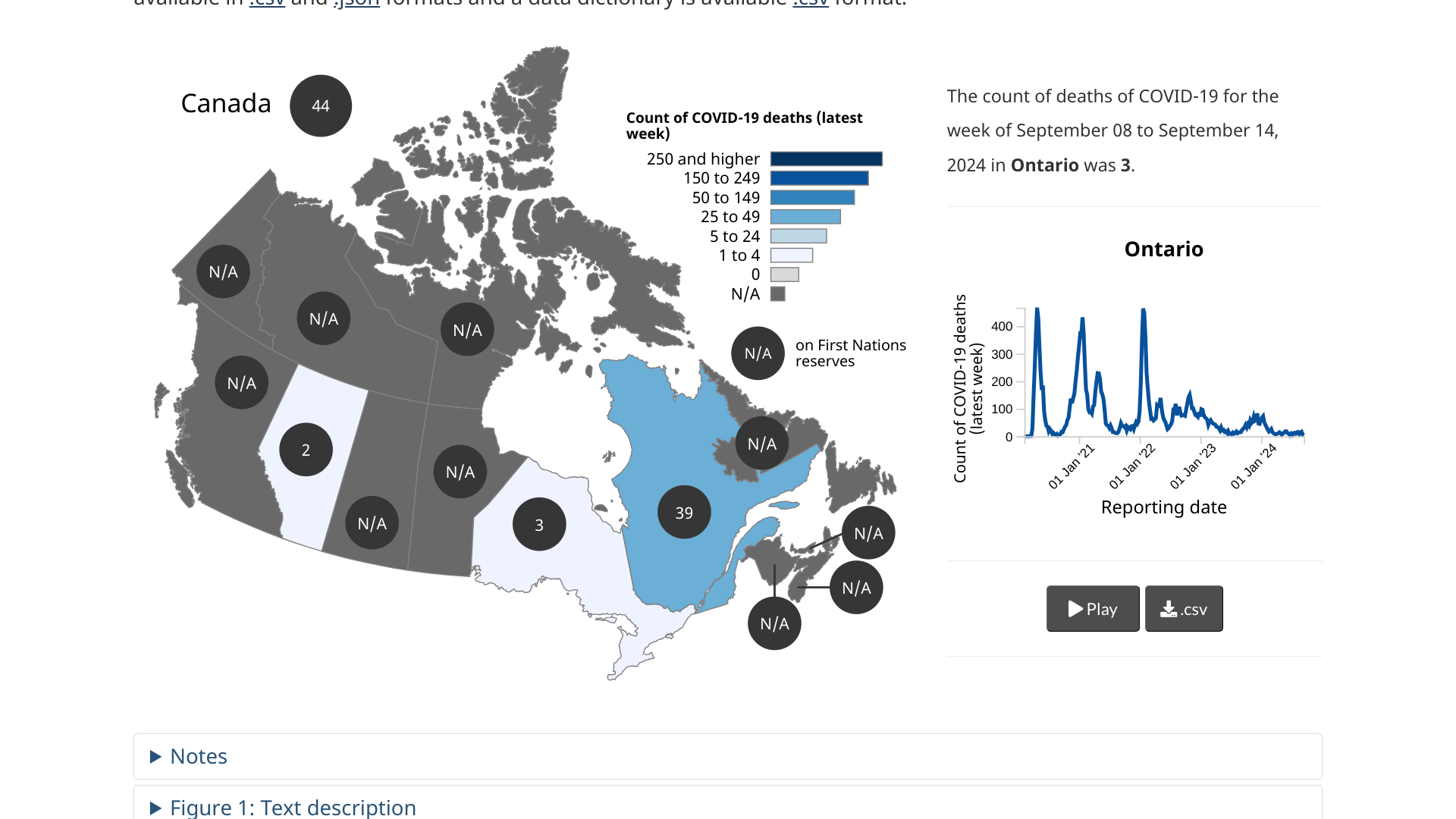
Health Canada posts a summary of COVID-19 cases, hospitalizations, and deaths across Canada as of September 24, 2024.
The U.S. Department of Health and Human Services (HHS), through the Administration for Strategic Preparedness and Response, announced today it is taking action to increase the supply of mpox vaccines.
This news supports the U.S. Government's commitment to making over 1 million combined doses of Bavarian Nordic A/S JYNNEOS® (MVA-BN®) vaccines available to the global mpox response focused in Africa.
Since JYNNEOS is a two-dose vaccination regimen, this announcement indicates about 500,000 individuals can be vaccinated.
“A public health threat to one is a public health threat to all. HHS is committed to fighting the current mpox outbreak, including through this vaccine donation. Disease doesn’t respect borders, and it is our duty to work together to make our world healthier. Our partnerships across the globe in fighting infectious disease will help keep us safe,” said HHS Secretary Xavier Becerra in a press release on September 24, 2024.
According to HHS, these vaccine efforts build on the $1.94 billion invested in funds and technical expertise to develop and sustain JYNNEOS, which is FDA-approved for both mpox and smallpox. The product would not exist without the investment and technical expertise the U.S. government provided.
More information on the U.S. response to the Clade 1 mpox outbreak can be found on the HHS mpox response website. JYNNEOS's efficacy against the Clade 1 mpox virus remains under investigation.