Search API
ImmunityBio, Inc. announced today that the drug substance had been completed and successfully qualified for “fill finish” (filling vials and finishing packaging), sufficient for 170,000 doses of 400mcg ANKTIVA®.
ANKTIVA (nogapendekin alfa inbakicept-pmln) is the first U.S. FDA-approved immunotherapy for non-muscle invasive bladder cancer that activates natural killer cells, T cells and memory T cells for a long-duration response.
Coupled with the recent announcement of a partnership with the Serum Institute of India for enhanced BCG vaccine availability, this provides the Company with a significant initial supply of ANKTIVA for commercial and clinical trial use in advance of the full operation of the Company’s drug substance and fill-finish manufacturing plants in California and New York.
In 2024, Anktiva will be priced at $35,800 per dose. The cost of the BCG vaccine is additional.
Since the Company’s merger with NantKwest in 2021, ImmunityBio has made significant capital investments in personnel, plants, and equipment to ensure the global capacity of the ANKTIVA drug product for both the commercial launch and clinical trials in bladder cancer and other tumor types in its pipeline.
Both drug substance and drug product facilities are nearing completion to ensure sufficient capacity and multiple GMP manufacturing sites for ANKTIVA in its approved indication and for clinical trials and future indications.
“Our belief in the importance of this molecule and its potential to evolve immunotherapy to the next level guided our strategic plan to invest for the future with anticipation of ANKTIVA’s approval,” said Rich Adcock, CEO & President ImmunityBio, in a press release on May 7, 2024.
“I’m grateful for our employees and our investors who have supported and believed in our commitment to invest for our long-term vision and future.”
The Company is applying its science and platforms to treating cancers, including developing potential cancer vaccines, immunotherapies, and cell therapies that we believe will sharply reduce or eliminate the need for standard high-dose chemotherapy.
TCompany says these platforms and their associated product candidates are designed to be more effective, accessible, and easily administered than current standards of care in oncology and infectious diseases.

Valneva SE today reported its financial results for the first quarter ending March 31, 2024, and provided corporate updates.
On May 7, 2024, the Company announced IXCHIQ®, the world's first and only licensed chikungunya vaccine, recorded initial sales of €0.2 million in the first quarter in the U.S.
In a press release, Peter Bühler, Valneva's Chief Financial Officer, commented, "The first quarter performance has been in line with our expectations. We are aiming to further capitalize on the travel industry recovery during the rest of the year (2024), including ramping up sales for IXCHIQ® to support our commercial sales growth while executing on our key R&D milestones."
IXCHIQ® is also under regulatory review in Canada, Brazil, and Europe, where it was granted accelerated assessment by the European Medicine Agency's Committee for Medicinal Products for Human Use. Decisions on these submissions are expected in 2024.
The U.S. Centers for Disease Control and Prevention (CDC) recently adopted the U.S. Advisory Committee on Immunization Practices recommendations, which include the chikungunya vaccine for persons aged ≥18 traveling to a country or territory with a chikungunya outbreak.
In addition, the CDC says the chikungunya vaccine may be considered for those traveling to a country or territory without an outbreak but with evidence of chikungunya virus transmission among humans within the last five years.
Persons aged >65 years, particularly those with underlying medical conditions, are likely to have at least moderate exposure* to mosquitoes OR Persons staying for a cumulative period of 6 months or more. Moderate exposure could include travelers with at least two weeks (cumulative) of exposure to mosquitoes in indoor or outdoor settings.
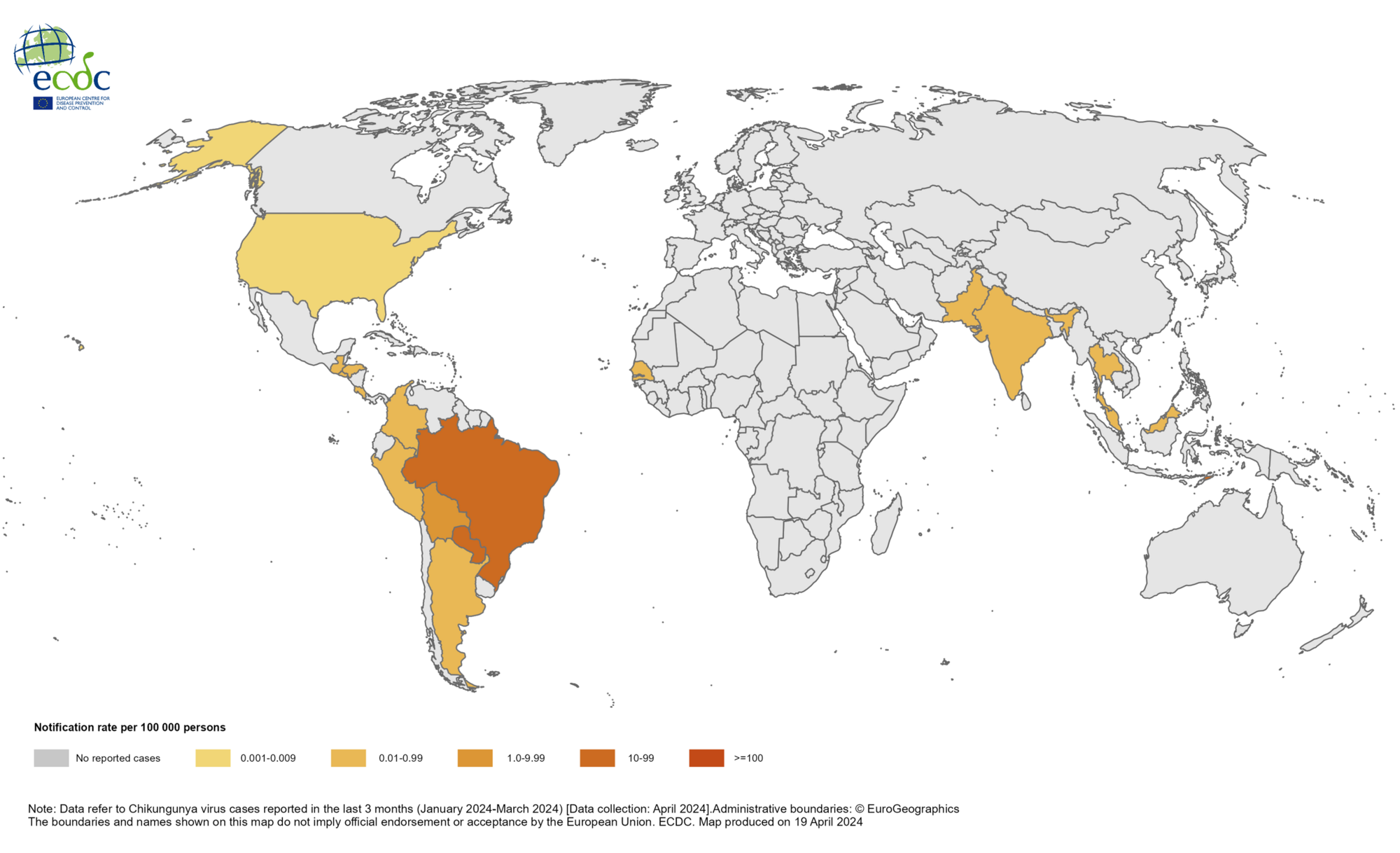
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes infected with the chikungunya virus.
As of May 2024, the World Health Organization says chikungunya was identified in nearly 115 countries, primarily in the Region of the Americas.
According to the European Centre for Disease Prevention and Control, approximately 70,000 chikungunya cases and 15 deaths have been reported worldwide in 2024.
New York City's Deputy Commissioner, Division of Disease Control, Celia Quinn, MD, MPH, issued a statement confirming that mpox continues circulating in the city.
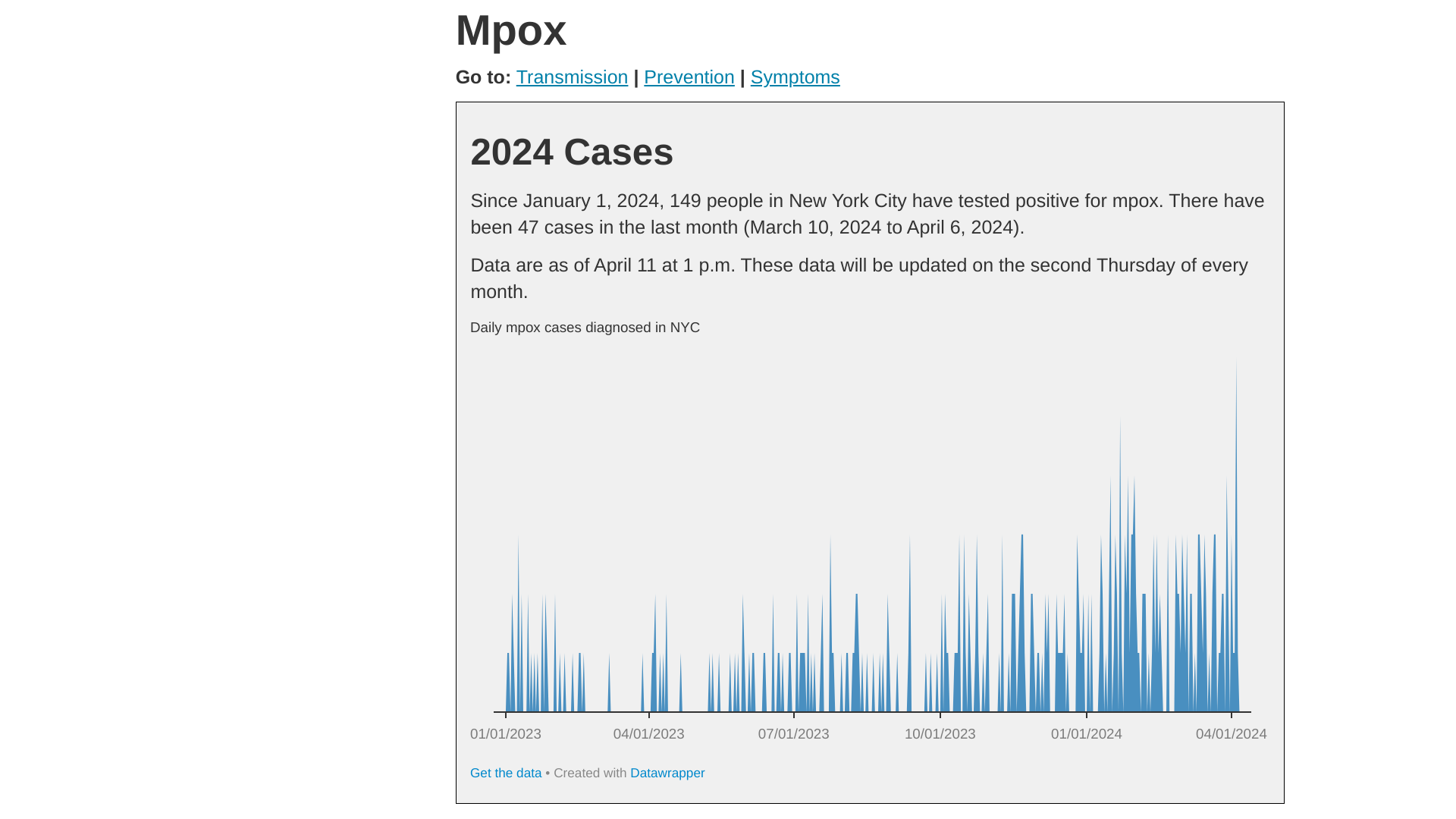
As of May 3, 2024, Health Advisory #12 reported the overall number of mpox cases is low compared to the 2022 outbreak. For most of 2023, the NYC averaged about 2 to 20 monthly cases.
Since October, there has been an average of 36 monthly cases, with a peak count of 51 in January 2024.
Of the 256 cases from October 2023 through April 15, 2024:
- 73% (188) were not vaccinated or had received only one dose,
- 94% were among men,
- 3.9% (10) of the infected people were hospitalized,
- Most were Black or Hispanic and between the ages of 25-44.
NYC confirmed the commercialization of the U.S. FDA-approved JYNNEOS® Mpox Smallpox vaccine is underway. As of May 2024, healthcare providers can order JYNNEOS through their distribution partners to make it available for at-risk individuals at local pharmacies.
Mpox is a contagious disease caused by the monkeypox virus. The U.S. Centers for Disease Control and Prevention and NYC continue to report only Clade II mpox cases in 2024.
Last week, the U.S. Government Accountability Office noted in a May 2, 2024 report that the Strategic National Stockpile (SNS) wasn't clear on how and from whom to request mpox supplies, including causing confusion and delays in mpox vaccine deliveries.
About 30% of U.S. jurisdictions during the mpox outbreak said the process for requesting SNS inventory did not follow written guidelines.
BioNTech SE today reported financial results for the first three months of 2024, and provided an update on its corporate progress.
Total revenues reported were €187.6 million for the three months in 2024, compared to €1,277 million for the comparative period in 2023.
The Germany-based company stated the year-over-year change was mainly due to lower commercial revenues from the sales of BioNTech’s COVID-19 vaccine worldwide, resulting from endemic-level demand for COVID-19 vaccines.
“In the past weeks, we have reported positive preliminary data for our individualized and off-the-shelf mRNA-based candidates, which further underline the potential of our iNeST and FixVac platforms. We look forward to providing more updates this year across our oncology portfolio, including our bispecific antibody and ADC programs,” said Prof. Ugur Sahin, M.D., CEO and Co-Founder of BioNTech, in a press release on May 6, 2024.
“In the remainder of the year, we plan to develop and commercialize a variant-adapted COVID-19 vaccine and accelerate our clinical development activities towards realizing the full potential of our oncology pipeline with a view to becoming a commercial company with marketed medicines for cancer and infectious diseases.”
BioNTech and Pfizer developed, manufactured, and delivered their Omicron XBB.1.5-adapted monovalent Comirnaty COVID-19 vaccine, which has received multiple regulatory approvals, including full approvals, authorizations for emergency or temporary use, or marketing authorizations, in more than 40 countries and regions.
BioNTech says it is now focused on preparing for variant strain vaccine adaptation to be ready for commercial launch ahead of the upcoming 2024/2025 vaccination season, pending approvals.

It has been about three decades since the U.S. Food and Drug Administration approved the first monoclonal antibody. Since then, antibody engineering has dramatically evolved.
The recent pandemic was the first time monoclonal antibody-based therapies were produced in significant quantities to combat a new infectious disease. Globally, clinics administered hundreds of thousands of antibody injections over the first two years of the pandemic.
Antibody therapy worked... until it didn't.
The U.S. CDC says the infectious virus's rapid evolution outpaced the benefits derived from antibodies.
According to Michael Dumiak's article published by IAVI on April 25, 2024, this experience and other issues have researchers assessing the future of antibody therapies for treating or preventing infectious diseases, including some of the most complicated pathogens, such as HIV and antibiotic-resistant bacteria.
A potential application is blocking mother-to-child transmission of HIV during birth and through the breastfeeding period.
"We are in the position that if you want more antibodies for infectious disease, you need to be very cautious," says Rino Rappuoli, scientific director of the Biotecnopolo di Siena Foundation in Italy.
The unedited, complete IAVI article is posted at this link.
Note: As of May 5, 2024, the U.S. FDA has not approved an HIV vaccine candidate.

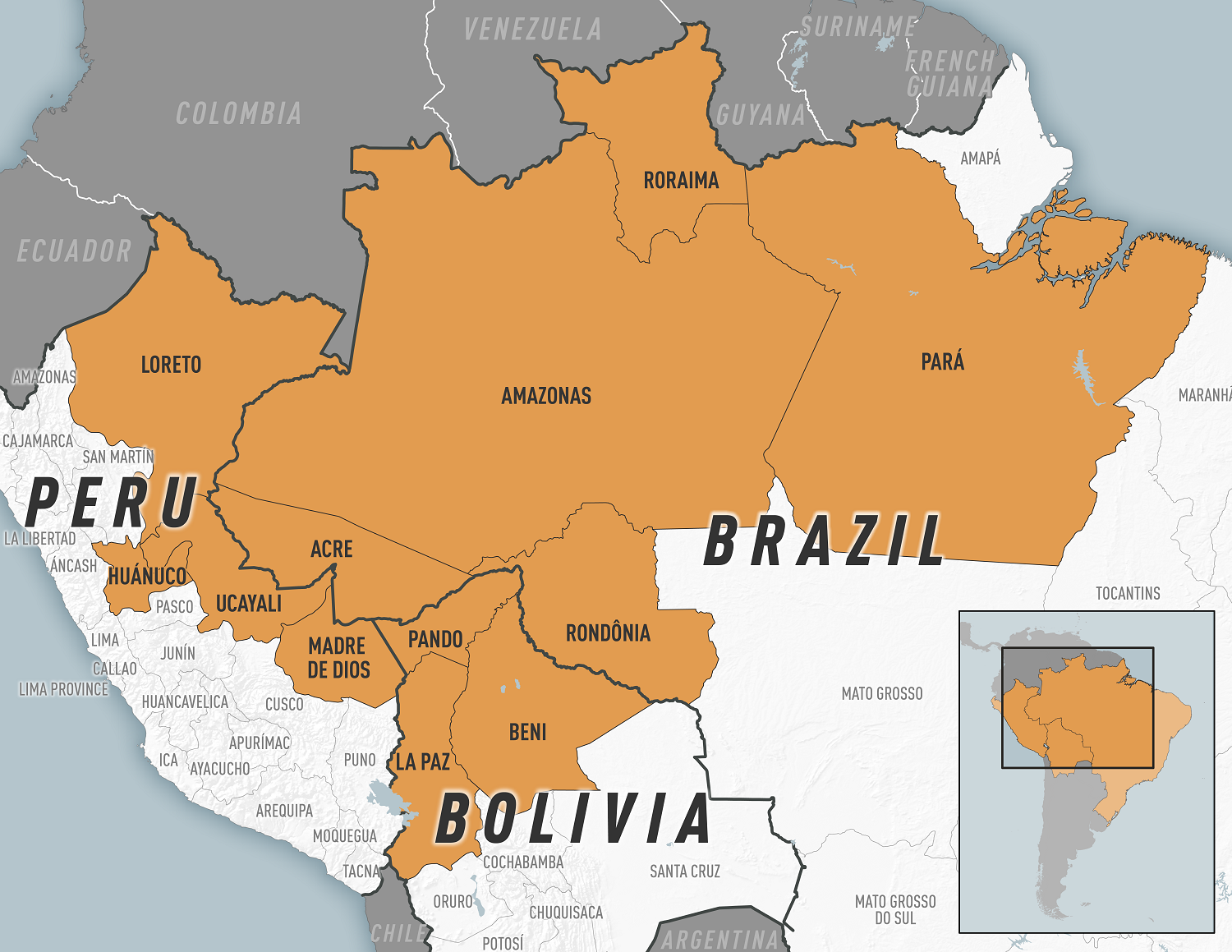
In 2024, there has been an increase in the detection of Oropouche fever outbreaks in areas of the Region of the Americas.
The U.S. Centers for Disease Control and Prevention (CDC) recently confirmed Oropouche fever outbreaks in parts of Brazil, Bolivia, and Peru.
For example, between 2023 and early 2024, 1,066 human cases of the Oropouche virus were registered in the Brazilian state of Amazonas.
To alert international travelers to this health risk, the CDC issued a Level 1, Practice Usual Precautions, Travel Health Advisory, saying Oropouche fever is spread through the bites of infected midges (flies) and Culicoides paraensis mosquitoes.
The illness is often mistaken for dengue.
The Pan American Health Organization (PAHO) says travelers to these areas should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
Most people recover without long-term effects.
Symptoms typically start 4–8 days after being bitten and last 3–6 days.
Oropouche fever has no cure or specific therapy, so treatment is symptomatic. Oral analgesics and anti-inflammatory agents can help with headaches and body pains.
As of May 2024, no licensed vaccines or specific antiviral treatments for Oropouche fever exist. However, recent clinical studies have used peptide vaccines to develop epitope-based vaccines, which could lead to potential use in the future.
New research presented at the Pediatric Academic Societies confirms rotavirus vaccines do not cause significant outbreaks of the disease in neonatal intensive care units (NICUs).
These researchers concluded that vaccine-strain rotavirus transmission in the NICU was rare and without clinical consequences.
The study found that 99.3% of non-vaccinated patients exposed to vaccinated patients did not test positive for the disease. Non-vaccinated patients who contracted rotavirus had no symptoms after 14 days.
Announced on May 3, 2024, these findings are significant because many NICUs avoid vaccinating against rotavirus due to a theoretical risk of transmission, yet some infants are too old to receive the vaccine once discharged from the NICU.
According to researchers, preterm infants are at higher risk of the highly contagious but preventable virus, yet few receive the vaccine in hospital settings.
This concern is because the rotavirus vaccine contains a weakened form of the virus.
“Immunization with rotavirus vaccine has been standard practice in the Children’s Hospital of Philadelphia NICU since 2007, and the safety of this practice was supported by retrospective clinical data published in Pediatrics in 2014 – however, this remains an uncommon practice in NICUs across the United States,” said Kathleen Gibbs, MD, the study’s lead neonatologist from Children’s Hospital of Philadelphia, in a press release.
“Our yearlong, prospective study, done in collaboration with the U.S. Centers for Disease Control and Prevention, suggests that the benefits of vaccinating NICU patients against rotavirus outweigh the risks. Inpatient vaccination allows protection of a vulnerable population against a common, preventable cause of severe diarrheal illness.”



