Search API
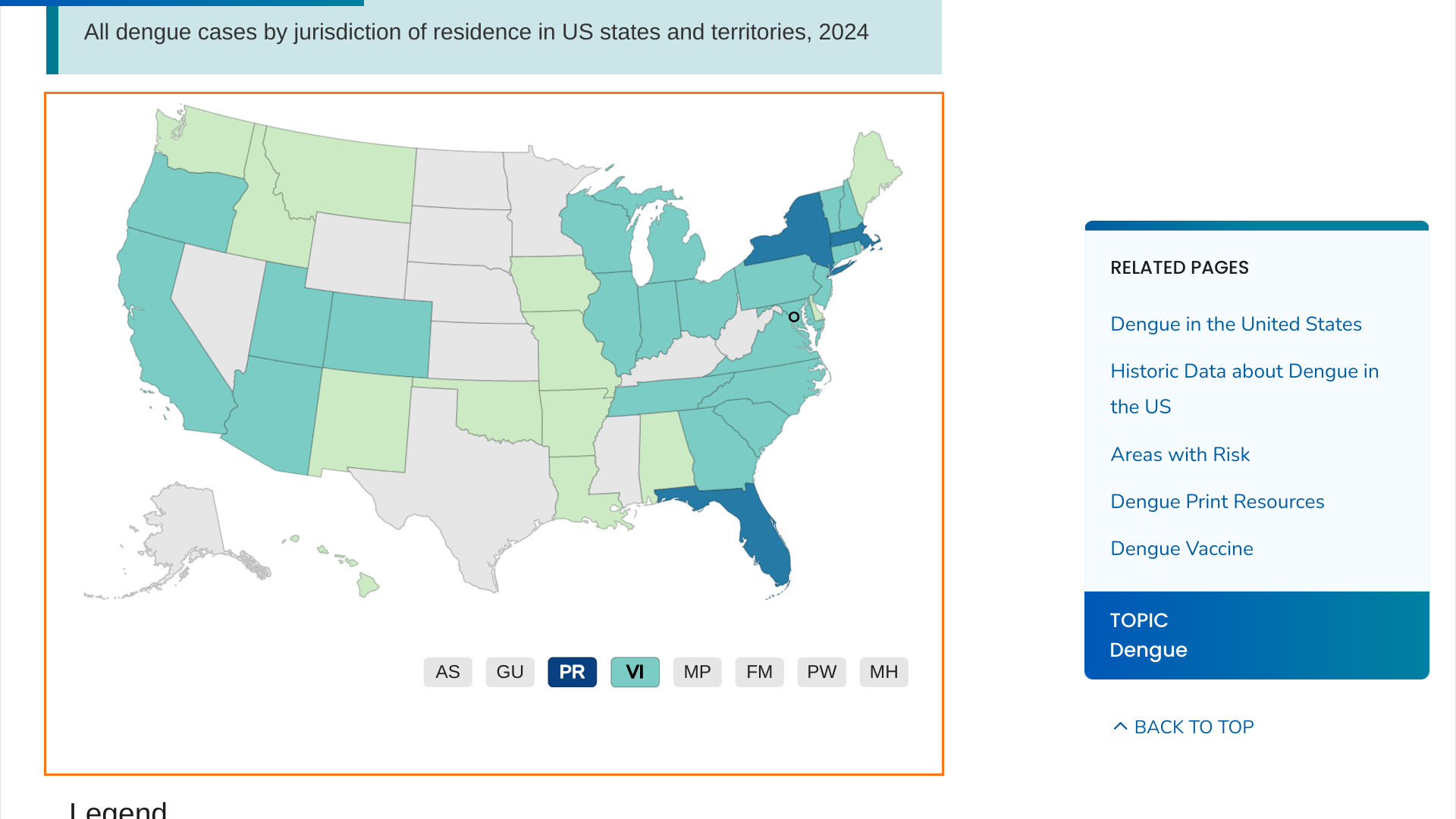
When the U.S. Centers for Disease Control and Prevention (CDC) published a Health Advisory (CDCHAN-00511) on June 25, 2024, it indicated that dengue fever posed a health risk in southeast Florida, New York, and Puerto Rico.
However, according to new CDC data, Massachusetts has reported 50 dengue cases this year.
While the CDC did not disclose whether these dengue cases were locally acquired, it can be assumed that they are travel-related since the mosquitoes that spread dengue are not found that far north in the United States.
However, countries in the Region of the Americas have reported a record-breaking number of dengue cases, exceeding the highest number ever recorded in a single year.
From a local guidance perspective, the Massachusetts Health Department (MDH) says there is no vaccine (Dengvaxia is no longer available in the U.S.); the best way to protect yourself is to not get bitten by mosquitoes.
MDH's website states if you have recently traveled to a region where mosquito-borne diseases are common and have any related symptoms, you should call your healthcare provider immediately and explain your travel history and symptoms.
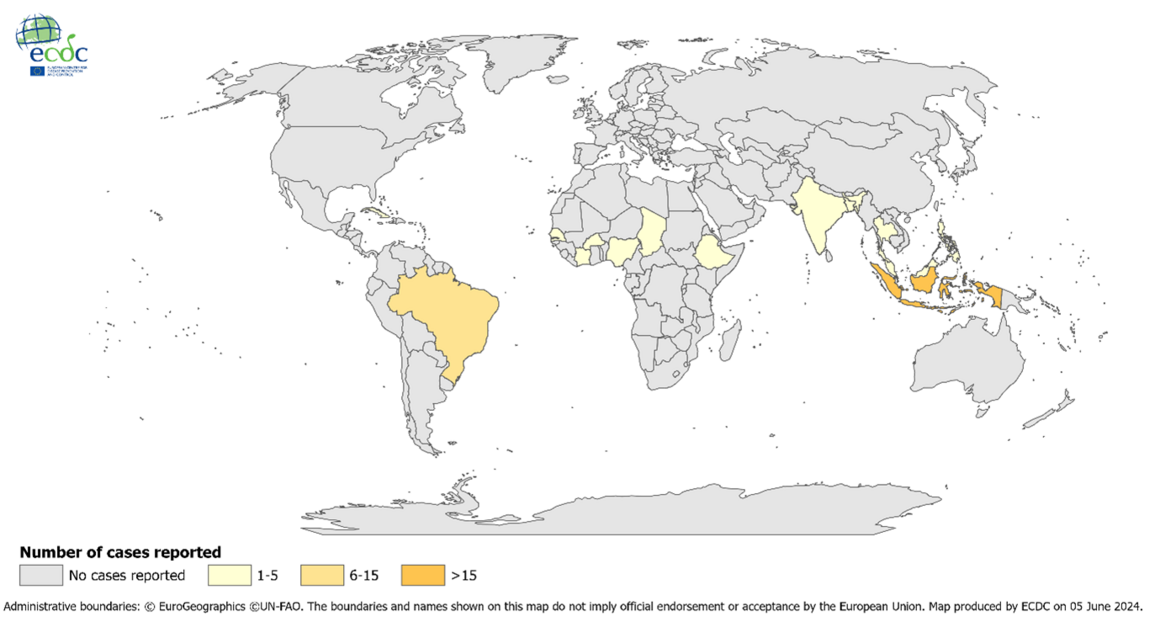
Valneva SE announced today that the European Commission (EC) has granted marketing authorization in Europe for the IXCHIQ® vaccine, which is used to prevent diseases caused by the chikungunya virus in adults.
IXCHIQ® is the world’s only licensed chikungunya vaccine.
The EC decision marks the third approval the Company has received for IXCHIQ® following approval from the U.S. FDA in late 2023 and Health Canada last month.
On July 1, 2024, Valneva stated it expects to deliver the first vaccine doses in Europe in the fourth quarter of 2024.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, “The EC approval marks a crucial milestone toward making this vaccine available to as many European citizens as possible .... It is critical to provide a vaccine solution not only to European travelers going to endemic chikungunya areas, such as South America or Africa.
Valneva has also submitted a Marketing Authorization Application to the UK Medicines and Healthcare products Regulatory Agency and the Brazilian Health Regulatory Agency, with potential approval in 2024.
Since the start of 2024, the Democratic Republic of Congo (DRC) has reported over 20,000 mpox cases, with more than 1,000 deaths, primarily affecting children.
In June 2024, the U.S. CDC issued a Level 2 Alert reporting a mpox outbreak in 25 out of 26 DTC provinces, including urban areas.
According to media sources, authorities in the DRC have responded to this outbreak by approving the use of two new vaccines.
AfricaNews.com reported on June 28, 2024, that emergency use authorization had been issued for the Jynneos® vaccine, developed by Bavarian Nordic, and LC16, produced by KM Biologics.
LC16 is a 3rd generation, live attenuated vaccine containing live vaccinia virus (LC16m8 strain) used to prevent smallpox and mpox.
The DRC decision follows rigorous evaluation by relevant authorities and stakeholders involved in the authorization process.
JYNNEOS (MVA-BN®, IMVAMUNE®) is a two-dose vaccine based on a live, attenuated vaccinia virus, Modified Vaccinia Ankara, and has been offered in the United States since May 2022.

PharmaJet® today announced that their Tropis® Intradermal (ID) Needle-free System will be used in a house-to-house polio immunization campaign.
Over a quarter million PharmaJet’s needle-free intradermal syringes have been provided to support this initiative.
The campaign will be conducted in two rounds to reduce the immunity gap significantly against type-2 poliovirus. Young children will receive the needle-free polio vaccine and novel oral polio vaccine (nOPV2) to achieve 95% coverage in each round.
The polio campaign, a collaboration of the African Field Epidemiology Network, WHO, UNICEF, BMGF, GAVI, and U.S. CDC, targets over 170,000 children in Somalia.
The most recent evidence for human circulating vaccine-derived polio virus-2 was in March 2024.
Through the Somalia Emergency Action Plan, the country will continue to work with humanitarian partners to reach about 1.5 million zero-dose children, most of whom live in the country’s highly populated central and southern areas.
Paul LaBarre, Vice President of Global Business Development at PharmaJet, commented in a press release on June 27, 2024, “In Somalia, we are eager to build on previous house-to-house campaign experience that demonstrates how needle-free enables vaccination teams to move quickly and achieve high coverage without the burden of sharps waste management and with reduced vaccine volume and cold chain logistics.”
The U.S. CDC reissued a Global Polio Alert on May 23, 2024, regarding polio outbreaks and poliovirus detections in 34 countries. The CDC recommends that visitors to these countries be fully vaccinated against polio.

As the three-day Advisory Committee on Immunization Practices (ACIP) meeting ended today, the morning session focused on Respiratory syncytial virus (RSV), the leading cause of hospitalization among U.S. infants.
Led by Sarah S. Long, MD, the Maternal/Pediatric RSV Work Group presentations included the summary of the effectiveness of Beyfortus™ (nirsevimab) in infants.
Beyfortus is a single-dose, extended half-life monoclonal antibody (mAb) that offers passive immunization to prevent lower respiratory tract infections. It has been approved by the U.S. FDA and other health agencies.
On June 28, 2024, Amanda Payne, PhD, MPH, stated that Beyfortus was about 80% effective against RSV-associated encounters and hospitalizations among infants in their first RSV season during the 2023-2024 RSV season.
Furthermore, the U.S. CDC's RSVVaxView recently reported that among females with a young infant, over 43% reported that their infant received Beyfortus.
The ACIP group, which comprises vaccine experts, loudly expressed its enthusiasm for the effectiveness and uptake of this first-year mAb therapy.
The group's primary concern was product availability for the 2024-2025 RSV season.
While Beyfortus was available in the U.S. for the 2023-2024 RSV season, demand quickly outstripped supply.
Beyfortus's producers, Sanofi and AstraZeneca, confirmed on May 2, 2024, that the expansion of the manufacturing network is progressing. In late 2024, the companies could have more than tripled their manufacturing capacity and increased mAb supply.
Of note, should Beyfortus production fall behind demand during the next RSV season, the U.S. FDA has approved a vaccine that pregnant women can receive, which enables antibodies to be passed to the unborn child.

Dynavax Technologies Corporation today announced that the first participant has been dosed in a Phase 1/2 clinical trial evaluating the safety, tolerability, and immunogenicity of Z-1018, the company's investigational vaccine candidate being developed for the prevention of shingles (herpes zoster).
The Phase 1/2 randomized, active-controlled, dose escalation, multicenter trial is expected to enroll approximately 440 healthy adults aged 50 to 69 years at trial sites in Australia and will evaluate the safety, tolerability, and immunogenicity of Z-1018 compared to the Shingrix® vaccine.
Key objectives of the trial include selecting the optimal glycoprotein E (gE) protein dose level and dosing schedule for further clinical development. The Phase 1/2 trial will also support the validation of a Patient-Reported Outcome measurement tool to differentiate Z-1018 on tolerability and support potential label claims.
"We believe there is an opportunity to develop an improved shingles vaccine with a significantly better tolerability profile than the market-leading shingles vaccine. One of the unique advantages of our vaccine candidate is CpG 1018 adjuvant's established safety and tolerability profile, combined with its ability to induce strong CD4+ T-cell responses, which are thought to be critical in preventing the reactivation of the herpes zoster virus," said Rob Janssen, M.D., Chief Medical Officer of Dynavax, in a press release on June 27, 2024.
Dynavax anticipates reporting top-line immunogenicity and safety data in the second half of 2025, including comparing CD4+ T-cells one month after the second of two vaccine doses.
According to the U.S. CDC, shingles risk increases with age and in people with weakened immune systems. About 33% of people in the United States develop shingles at least once, and fewer than 100 people die of shingles each year.
As of June 2024, there are four approved shingles vaccines and several vaccine candidates conducting clinical research.

While the first day of the Advisory Committee on Immunization Practices (ACIP) meeting was focused on Respiratory Syncytial Virus vaccines, day #2's agenda focuses on respiratory diseases.
On June 27, 2024, the U.S. CDC's vaccine committee meeting agenda includes presentations on updated COVID-19, influenza vaccine options, and a new pneumococcal vaccine (PCV21).
With the 2024-2025 respiratory season fast approaching in the Northern Hemisphere, the general public can listen to today's clinical discussions and votes at this YouTube link.
The ACIP develops recommendations for U.S. immunizations, including ages when vaccines should be given, number of doses, time between doses, and precautions and contraindications.
If the recommendations are adopted by the CDC Director Mandy K. Cohen, MD, MPH, they will be published in a CDC MMWR.