Search API
Today marks the official rollout of the newly approved R21/Matrix-M™ malaria vaccine, co-developed by the University of Oxford and Serum Institute of India (SII), which has committed to producing 100 million vaccines.
This malaria vaccine utilizes Novavax’s Matrix-M™ adjuvant technology.
The first official vaccination is scheduled for July 15, 2024, in Abidjan, Côte d’Ivoire, and it will be subsequently introduced in 38 districts across the country.
According to a press release, 15 African countries will introduce malaria vaccines with Gavi support in 2024. These countries plan to reach around 6.6 million children with the malaria vaccine in 2024 and 2025.
John Jacobs, President and Chief Executive Officer of Novavax Inc., commented, "The introduction of the R21/Matrix-M™ malaria vaccine in Côte d'Ivoire marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health."
"Novavax is proud of the contribution of our Matrix-M™ adjuvant in this vaccine and in making this moment possible, and value our continued collaboration with the University of Oxford and SII, as well as the lifesaving work of WHO, Gavi, and UNICEF.”
R21/Matrix-M is a low-dose, highly effective, and affordable vaccine that can be manufactured quickly and scale. Ghana, Nigeria, Burkina Faso, and the Central African Republic have approved the new vaccine, and many others are preparing to receive shipments.
Malaria vaccines are currently unavailable in the United States.
Novavax, based in Gaithersburg, MD., U.S., promotes improved health by discovering, developing, and commercializing innovative vaccines to help protect against serious infectious diseases.
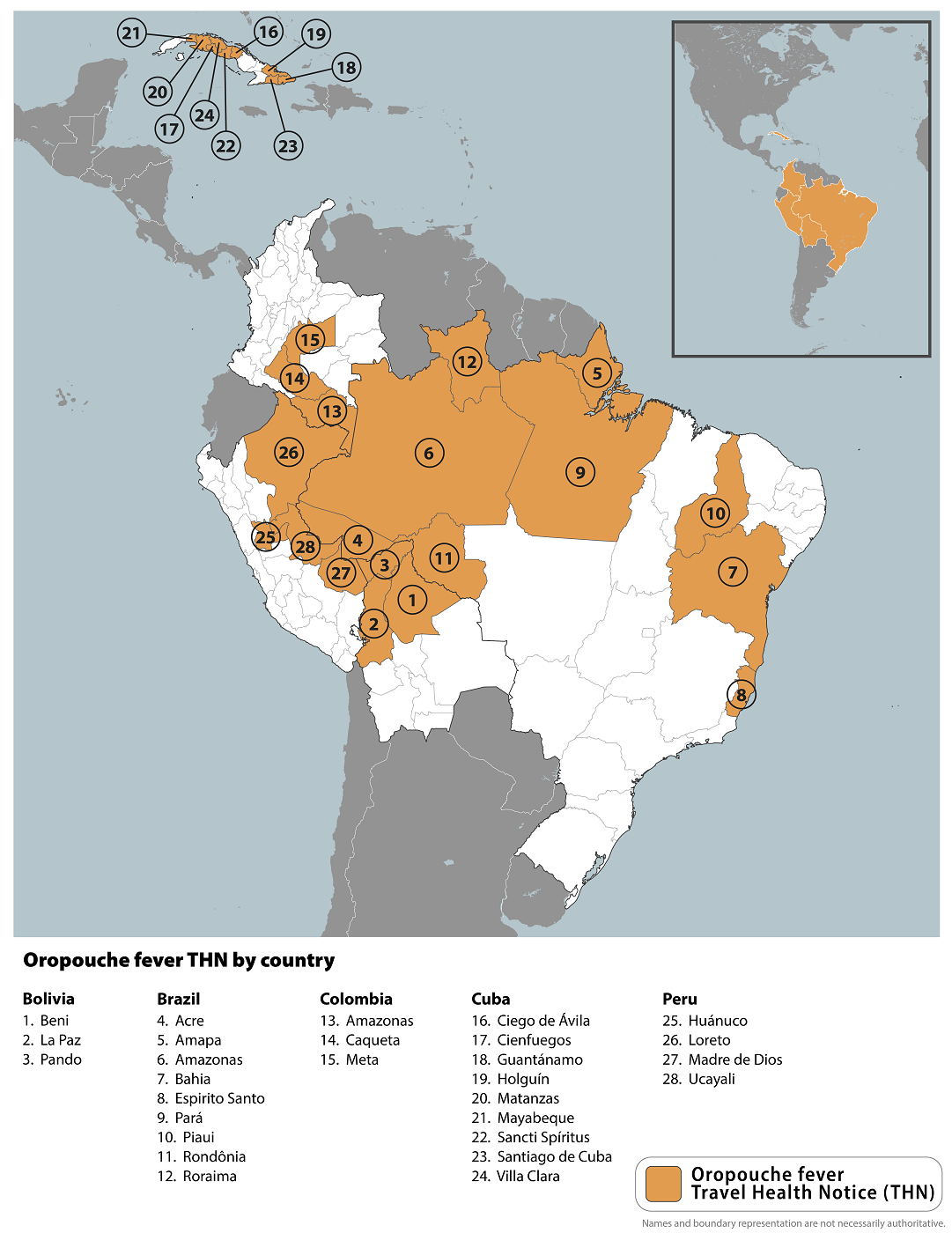
The U.S. CDC reissued a global warning about Oropouche fever outbreaks in various communities of Brazil, Bolivia, Colombia, Peru, and Cuba in the Region of the Americas.
In late June 2024, the CDC said travelers should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
For example, the Ministry of Public Health of Cuba reported the first-ever outbreak of Oropouche virus disease in May, confirming 74 cases from the Province of Santiago de Cuba (54) and the Province of Cienfuegos (20).
Oropouche virus is primarily transmitted to humans through the bite of the midge, Culicoides paraensis, but the mosquito Culex quinquefasciatus can also transmit it.
The Pan-American Health Organization / World Health Organization urges Member States to intensify surveillance given its clinical presentation and considering the current situation of chikungunya, dengue, zika, and other common vector-borne diseases in the Region.
While there are approved vaccines to prevent chikungunya and dengue diseases, Oropouche and zika viruses do not have vaccines available in July 2024.
The board of directors of CSPC Pharmaceutical Group Limited announced on July 11, 2024, that the mRNA Respiratory Syncytial Virus (RSV) vaccine candidate (SYS6016) has obtained approval from the National Medical Products Administration of the People’s Republic of China to conduct human clinical trials in China.
Currently, there is no vaccine available in China that protects people from RSV infection.
In preclinical studies, SYS6016 translated into the prefusion conformation F-protein in vivo and induced high titers of long-lasting neutralizing antibodies.
CSPC wrote that this vaccine candidate exhibits good protection against RSV-A and RSV-B subtype viral strains and has a good safety profile.
CSPC confirmed it would endeavor to advance the clinical research and market SYS6016 as soon as possible to create value for society and shareholders.
As of July 13, 2024, three RSV vaccines and one monoclonal antibody for infants (Beyfortus) were approved for use in the United States.

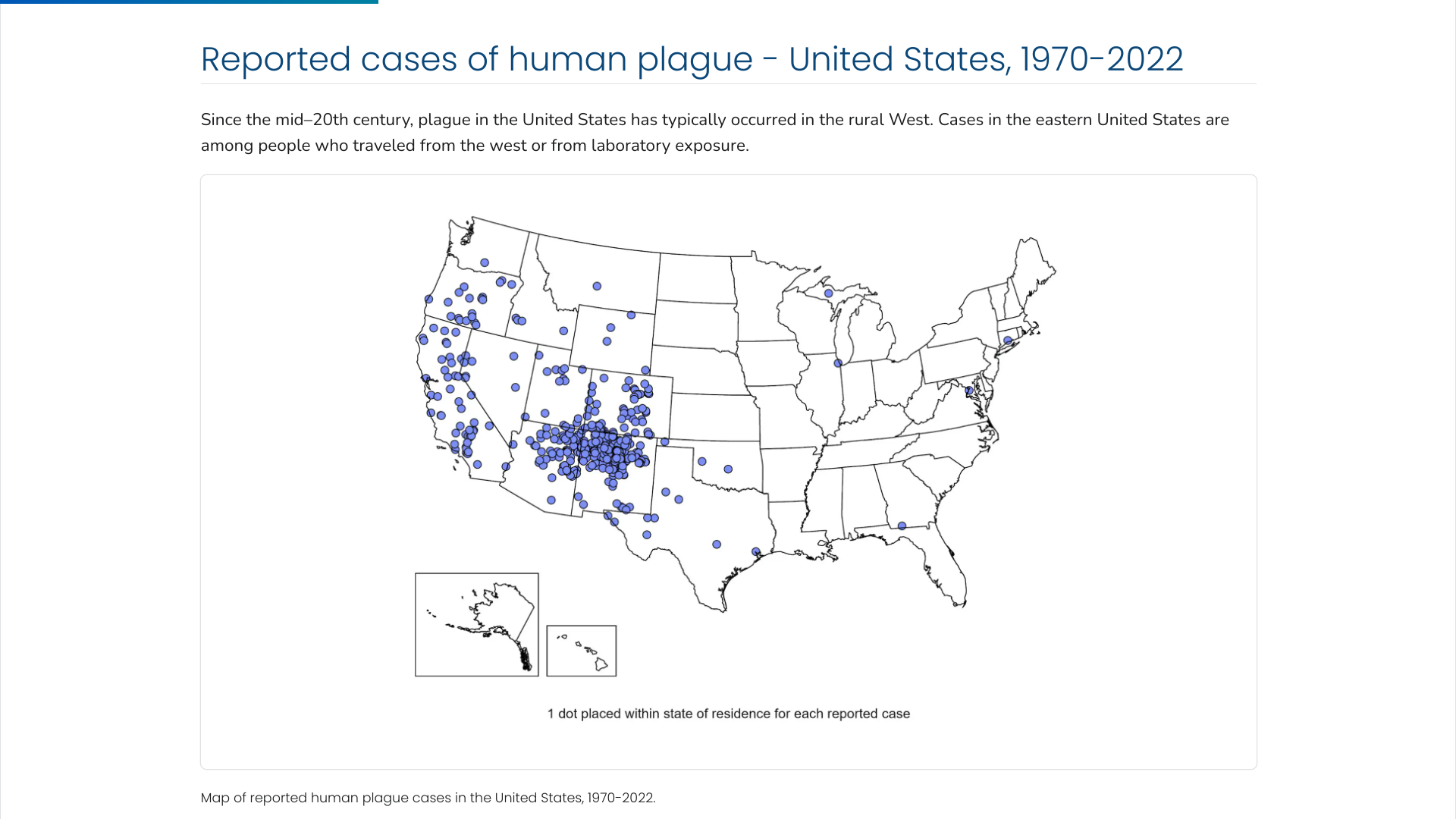
The U.S. Centers for Disease Control and Prevention (CDC) says the Plague was first introduced into the United States in 1900. The plague bacterium (Yersinia pestis) is transmitted by fleas and cycles naturally among wild rodents.
Over the decades, the Plague spread from urban rats to rural rodent species and became entrenched in many areas of the western U.S.
Almost all of the cases reported in the last 20 years have occurred among people living in small towns and villages or agricultural areas rather than in larger towns and cities, says the CDC.
As of 2024, the CDC estimates that seven human cases of Plague occur in the U.S. each year.
Recent plague cases include the Pueblo Department of Public Health and Environment confirming a human case of Plague in a Pueblo County resident on July 9, 2024.
And in February 2024, health officials in Oregon reported a case of bubonic Plague in a resident who they said likely contracted it from a pet cat.
Globally, the most human plague cases since the 1990s have occurred in Africa.
From a prevention perspective, plague vaccines are no longer available in the U.S. However, plague vaccine candidates are in development but are not expected to be commercially available in the immediate future.
In March 2023, the first mRNA-based, lipid nanoparticle vaccine was found effective against lethal bacteria in mice.
As numerous vaccine manufacturers strive to produce improved flu shot options, innovative influenza vaccine candidates post positive results in 2024.
Intranasal vaccines are known to provide a wall of defense at the site of infection, helping prevent influenza viruses from entering the nasal mucosa.
FluGen, Inc., today announced the results of its study comparing the coadministration of intranasal M2SR and the high-dose flu shot in older adults ages 65-85.
Published in the journal Lancet Infectious Diseases on July 11, 2024, the study, funded by the U.S. Department of Defense, evaluated the safety, tolerability, and immunological response to FluGen’s investigational supra-seasonal, live, single-replication, intranasal influenza vaccine when administered with Fluzone High Dose inactivated influenza vaccine.
These researchers concluded the H3N2 M2SR vaccine coadministered with Fluzone HD in older adults was well tolerated and provided enhanced immunogenicity compared with Fluzone HD administered alone.
This finding suggests the potential for improved influenza vaccination efficacy in this age group.
“The idea of delivering two vaccines in one sitting has become widely accepted,” said Paul Radspinner, President and Chief Executive Officer of FluGen, in a press release on July 12, 2024.
“Imagine being at your local pharmacy for your annual flu shot and also receiving a quick nasal spray that would greatly enhance your chances not only of becoming seriously ill but of being infected at all. This combination solution could have a tremendous impact on the health of older adults."
Radspinner went on to discuss the possible impact on influenza pandemic protection. “If H5N1 or any other mutating influenza strain were to begin infecting millions of people, imagine the benefits of combining an intranasal vaccine, which could stop most infections from occurring, with a strong antibody-based vaccine shot."
"The impact on human health could be unequaled in our history.”
As of July 2024, new trivalent influenza vaccines, such as Flucelvax, had been shipped to pharmacies before the 2024-2025 flu season launches in the United States.

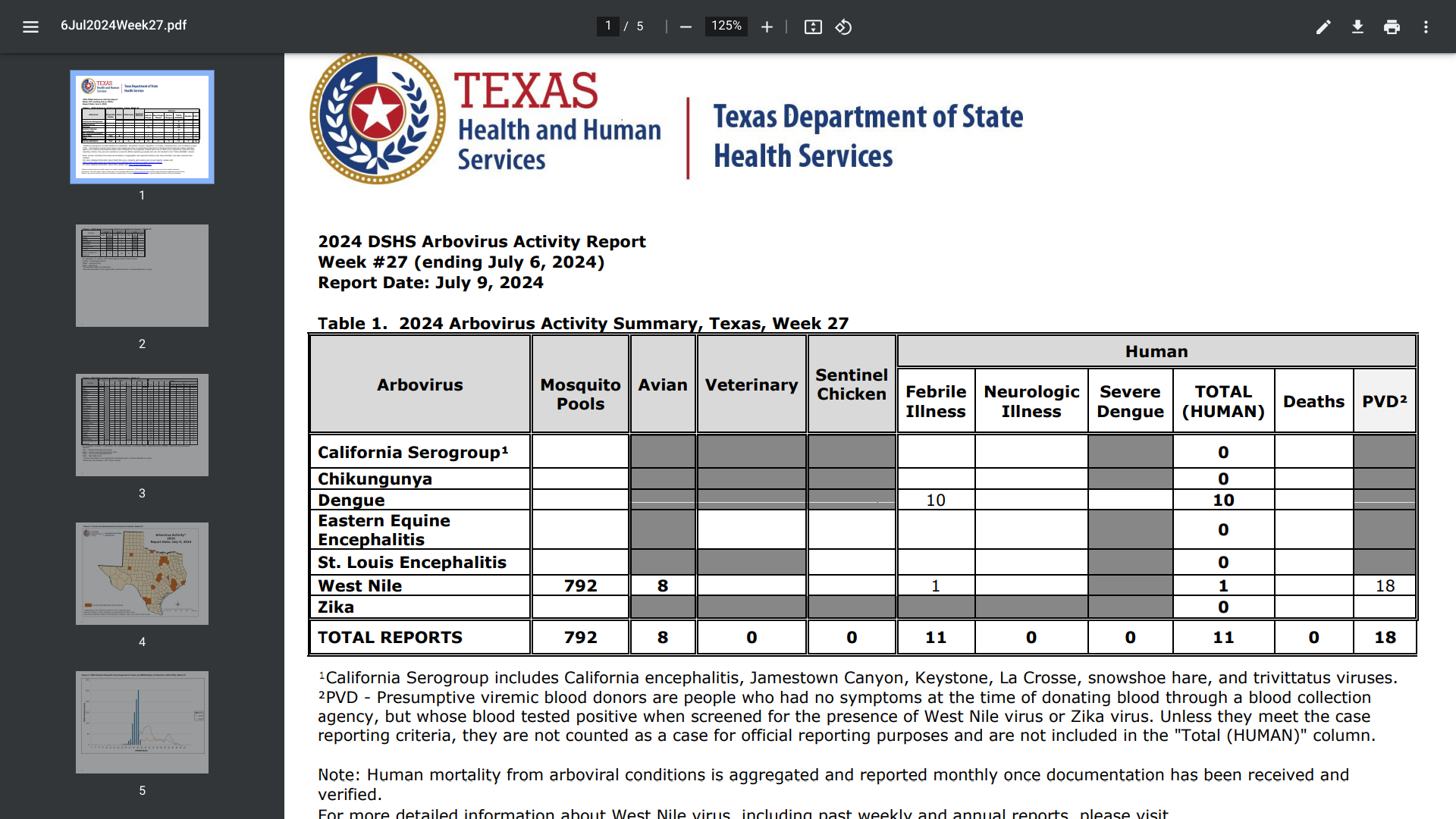
Texas public health officials announced today that they are urging Texans to protect against mosquito bites after confirming ten travel-associated dengue cases for 2024.
As of July 11, 2024, all of the dengue cases reported in Texas this year were acquired while traveling internationally.
However, a small number of dengue cases have been acquired from mosquito bites in southern Texas in recent years. Mosquitoes that carry the dengue virus are found in both Mexico and Texas.
The Texas Department of State Health Services (DSHS) confirmed the new dengue cases were reported in Collin, Dallas, Fort Bend, McMullen, Montgomery, and Travis counties.
In 2023, there were 79 cases of dengue in Texas, including one locally acquired case in Val Verde County.
To the south of Texas, Mexico has reported about 1,000 dengue cases in 2024. In 2023, Mexico reported over 277,000 dengue cases.
“Unfortunately, many mosquitoes in Texas can spread diseases, such as West Nile and dengue. These diseases are often mild, but some people will develop severe illness,” said DSHS Commissioner Jennifer Shuford, MD, MPH, in a press release.
According to DSHS, about 25% of dengue infections become symptomatic.
Most people recover completely within two weeks. However, about one in 20 symptomatic people develop a severe infection called Dengue Hemorrhagic Fever that can be fatal if untreated.
From a prevention perspective, two dengue vaccines are used in various countries but not in the U.S. as of July 11, 2024.
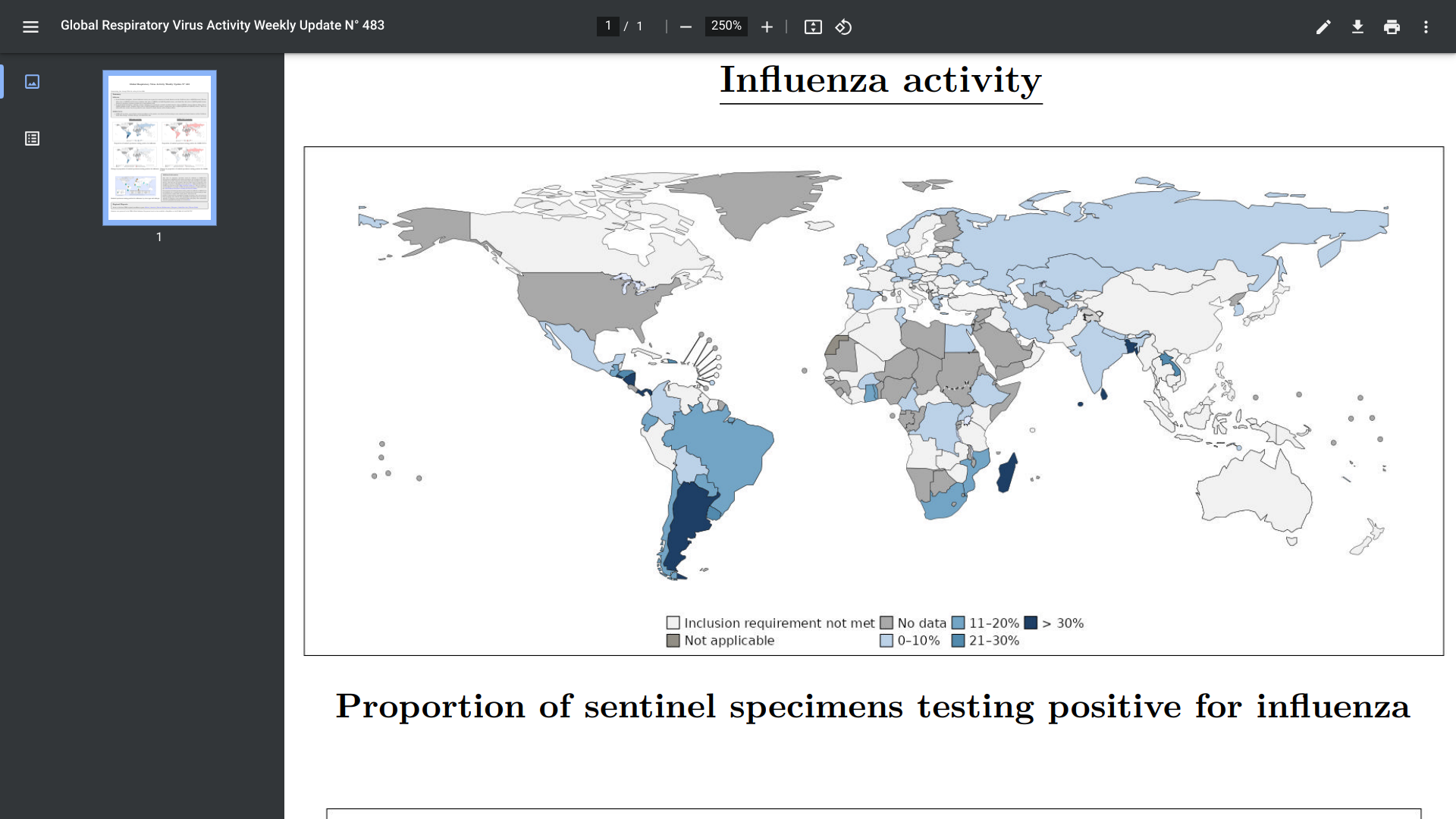
According to the World Health Organization (WHO) Influenza Update N° 483, elevated influenza activity was reported in countries in Central America and the Caribbean, Western Africa, Southern Asia, and South East Asia due to various virus types.
As of July 10, 2024, in the Southern Hemisphere, influenza activity continues to be elevated in South America and Oceania countries. There are indications that activity may have peaked in some South American and Southern African countries.
In the United States, influenza case reports for the 2024-2025 flu season are reduced.
However, flu shots for the new season have already been distributed to pharmacies in the U.S.