Search API
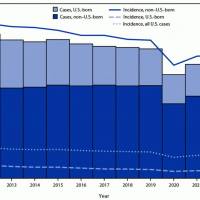
ImmunityBio, Inc. today announced its financial results for the third quarter ended September 30, 2024. The company achieved a net product revenue of approximately $6 million during the three months ended September 30, 2024, surpassing net product revenue of $1 million in the prior quarter and analyst estimates.
ANKTIVA® (N-803), U.S. FDA-approved and commercially available since May 2024, is now widely accessible to patients through commercial and government insurance programs (VA, DoD, Medicare). ImmunityBio has secured coverage for over 200 million medical lives through medical reimbursement policies.
ANKTIVA is a cytokine interleukin-15 (IL-15) that plays a crucial role in the immune system by affecting the development, maintenance, and function of key immune cells—NK and CD8+ killer T cells—that are involved in killing cancer cells.
By activating NK cells, ANKTIVA overcomes the tumor escape phase of clones resistant to T cells and restores memory T cell activity, resulting in a prolonged duration of complete response.
“The U.S. launch of ANKTIVA for non-muscle-invasive bladder cancer (NMIBC) CIS continues to gain momentum, and we are pleased to see the clinical impact for patients,” said Richard Adcock, President and CEO of ImmunityBio, in a press release on November 12, 2024.
“The Centers for Medicare and Medicaid Services have issued our permanent J-code, effective January 1, 2025."
"Our submission of ANKTIVA for NMIBC CIS to the U.K.'s MHRA for potential approval demonstrates our plans for global expansion. Further, we anticipate an EU submission this quarter.”

Arcturus Therapeutics Holdings Inc. today announced that the U.S. Food and Drug Administration (FDA) has issued a “Study Can Proceed” notification for the Company’s Investigational New Drug application, ARCT-2304, a self-amplifying mRNA (sa-mRNA) vaccine candidate for active immunization to prevent pandemic influenza disease caused by H5N1 virus.
The sa-mRNA vaccine candidate is designed to make many copies of mRNA within the host cell after intramuscular injection to enhance the expression of haemagglutinin and neuraminidase antigens, thereby enabling lower doses than conventional mRNA vaccines.
“Arcturus is actively engaged with the U.S. government to prepare for the next pandemic, and clearance to proceed into the clinic with our STARR® self-amplifying mRNA technology is a key step in this important process,” said Joseph Payne, President & CEO of Arcturus Therapeutics, in a press release on November 11, 2024.
“The Phase 1 clinical trial is designed to evaluate the safety, reactogenicity, and immunogenicity of ARCT-2304 as a potential vaccine to protect against the highly pathogenic H5N1 avian influenza.”
The clinical study is funded by the U.S. Biomedical Advanced Research and Development Authority.
The U.S. and European vaccine agencies have previously approved avian and pandemic influenza vaccines and have recently awarded funding grants for (bird flu) vaccine candidates.

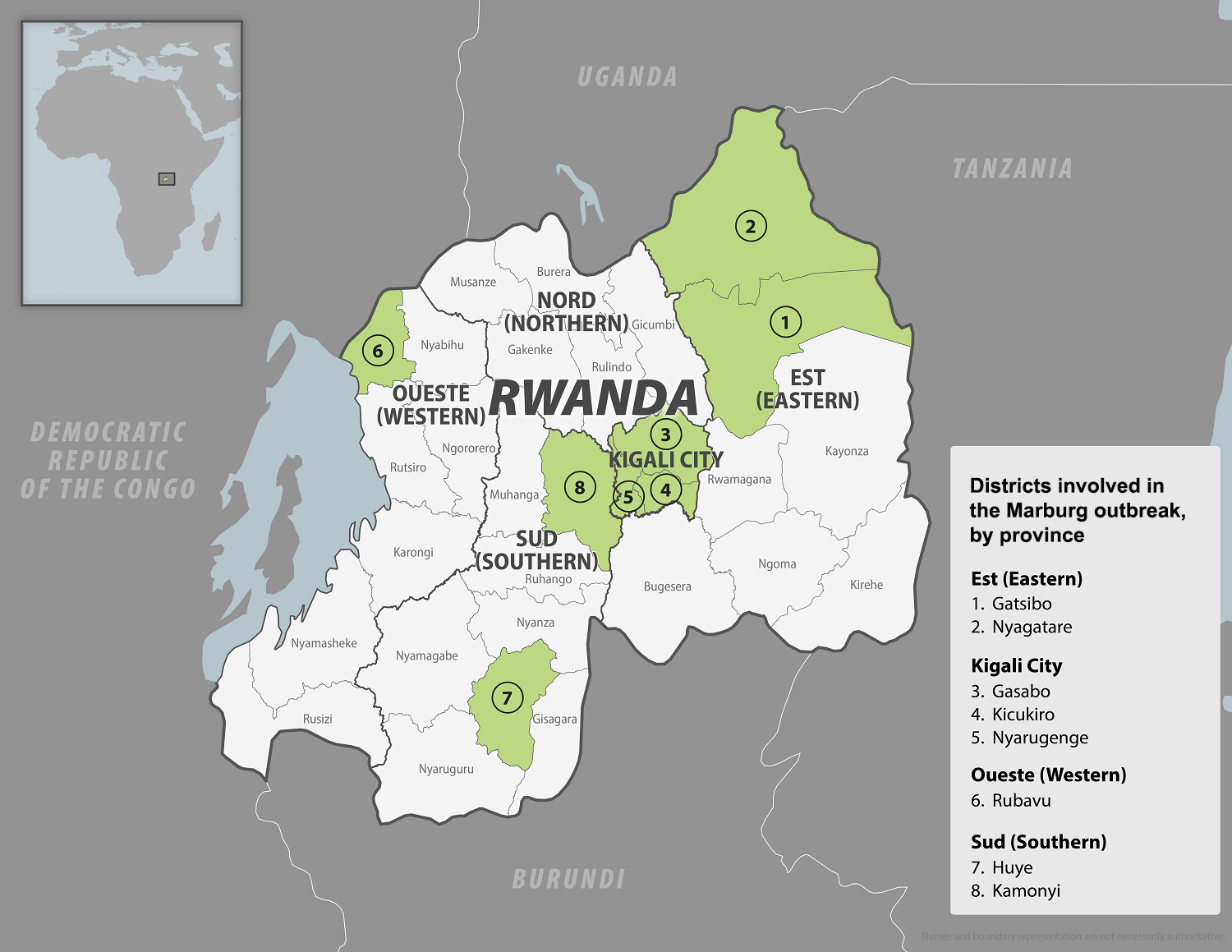
The WHO Africa recently announced that the Republic of Rwanda had discharged the last Marburg virus disease (MVD) patient, kicking off the mandatory 42-day countdown to declare the outbreak's end.
As of November 9, 2024, a total of 66 MVD cases and 15 deaths have been recorded during the outbreak, which was declared on September 27, 2024. Health workers, who constitute almost 80% of the cases, primarily became infected while providing emergency care to their colleagues and patients.
"This outbreak demonstrates that with the best available treatment, recovery is possible, and contributions to science can be made," said Dr Sabin Nsanzimana, Rwanda's Minister of Health, in a press release.
The World Health Organization published the Marburg vaccine development landscape on February 13, 2023. As of November 2024, no approved MVD vaccines exist.
Marburg is a highly virulent virus with a fatality ratio of up to 88%, and it was initially detected in Germany in 1967 following a lab incident. The virus belongs to the same family as the Ebola virus. Illness begins abruptly with high fever, severe headache, and malaise, and many patients develop severe hemorrhagic symptoms within seven days.
Currently, the U.S. CDC's Level 3—Reconsider Nonessential Travel Advisory remains active. The CDC recommends reconsidering nonessential travel to Rwanda, which is experiencing an outbreak of Marburg.
Novavax, Inc. today announced that the U.S. Food and Drug Administration (FDA) has cleared the Company to begin enrolling the planned Phase 3 clinical trial following the determination that Novavax satisfactorily addressed all clinical hold issues regarding its COVID-19-Influenza Combination and stand-alone influenza vaccine candidates.
"We thank the FDA for their partnership and thorough review of the additional information provided as part of our response package," said Robert Walker, MD, Chief Medical Officer, Novavax, in a November 11, 2024 press release.
"We plan to start our Phase 3 trial as soon as possible."
The Company did not estimate the availability of these innovative vaccine candidates.
Novavax is a global company advancing protein-based vaccines with its Matrix-M™ adjuvant, such as the R21/Matrix-M™ malaria vaccine currently available in Africa.

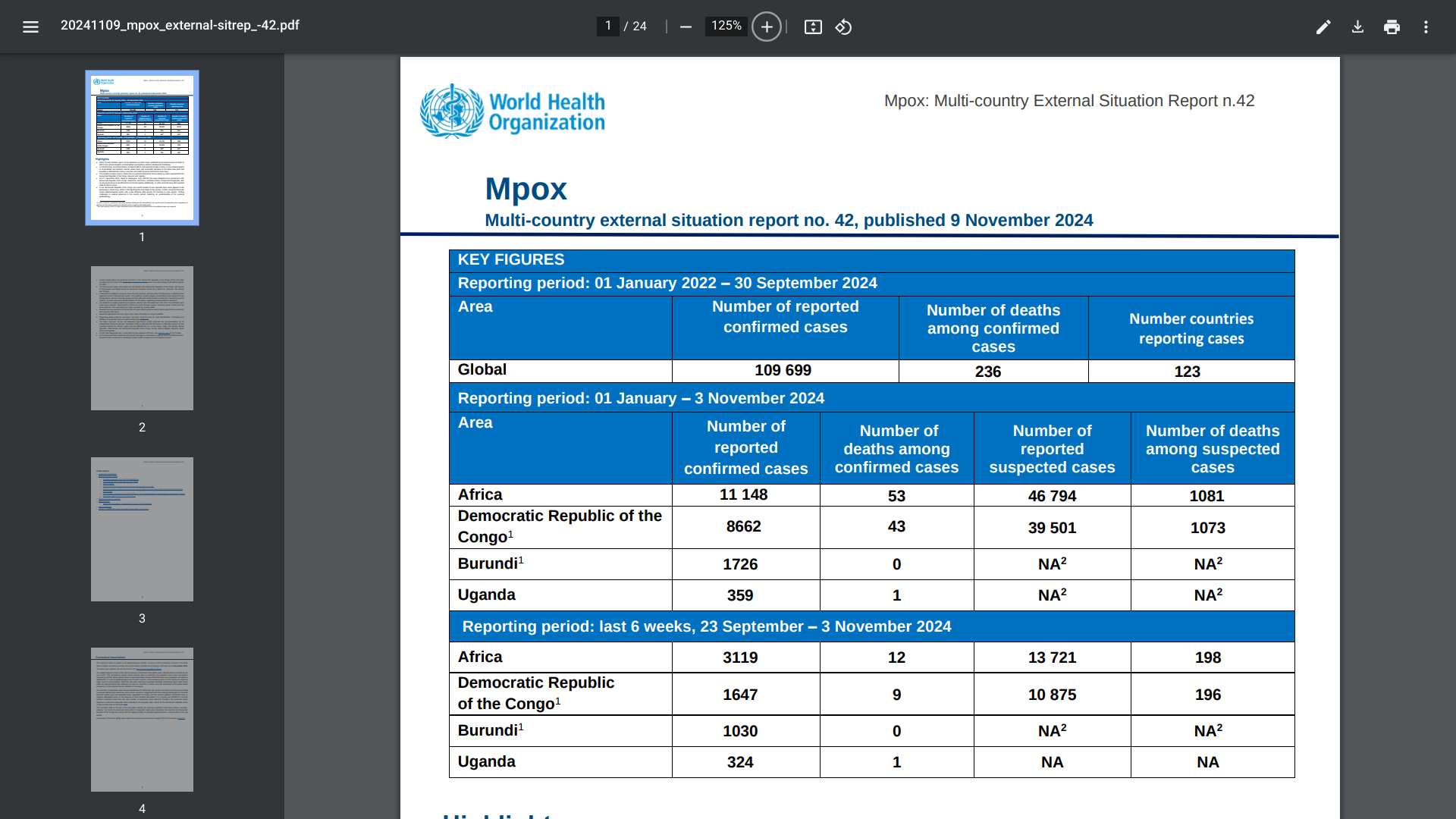
The World Health Organization (WHO) today published its 42nd situation report for the multi-country outbreak of mpox, which provides an update on the epidemiological situation of mpox in the WHO African Region and countries in the WHO Eastern Mediterranean Region.
The number of mpox cases in Africa is generally rising, driven mainly by cases reported from the Democratic Republic of the Congo (DRC).
As of November 11, 2024, the WHO confirmed Clade Ib monkeypox virus (MPXV) had been detected in six provinces in the DRC: South Kivu, North Kivu, Kinshasa, Kasai, Tshopo, and Tanganyika. Additionally, 11 other African countries have also reported clade Ib MPXV cases.
Since the last WHO situation report, three countries outside of Africa have confirmed travel-related cases of clade Ib MPXV,
For the first time, local transmission of clade Ib MPXV in the United Kingdom of Great Britain and Northern Ireland, where three (all) household members of the initial case (who had traveled to affected East African countries with clade Ib) were confirmed to have mpox.
Most impacted countries offer access to Bavarian Nordic's JYNNEOS® (MVA-BN®) vaccine to prevent mpox infections. The first round of mpox vaccination has concluded in the DRC, with around 51 500 people vaccinated across six provinces.
Furthermore, the clade 2 outbreak that began in May 2022 continues in numerous countries, including the United States.
Over the past few days, announcements have indicated more people may visit the Republic of El Salvador next year.
On November 1, 2024, Volaris El Salvador and Miami International Airport officials celebrated the launch of four weekly nonstop flights between San Salvador and Miami, Florida. In 2025, additional U.S. cities will also offer direct flights to this Central American destination.
On November 8, 2024, the U.S. Embassy in El Salvador reported significantly reducing gang-related activity and associated crime in the last two years. Recognizing these positive changes, the U.S. Department of State reduced its travel advisory to Level 2.
To keep visitors informed of local issues, the State Department recommends using major highways and roads and minimizing travel outside metropolitan areas when visiting El Salvador. It also recommends that visitors enroll in the Smart Traveler Enrollment Program to receive alerts and make locating you in an emergency easier.
From a health perspective, the U.S. CDC says dengue outbreaks are a year-round risk in many parts of the world, including El Salvador. As of November 2024, over 7,200 dengue cases were reported, an increase from the 5,788 cases confirmed last year.
Additionally, chikungunya, another mosquito-transmitted disease, has been confirmed in 47 people this year.
"For U.S. travelers with plans to visit El Salvador, it's essential to receive the hepatitis A and typhoid fever vaccines before visiting because you'll want to sample many of the excellent Salvadorian foods while on vacation. Be sure you're up-to-date on all routine vaccines, like hepatitis B, flu, measles (MMR), and tetanus," Jeri Beales, MSN, R.N., informed Vax-Before-Travel.
"Mosquitos are also a problem throughout El Salvador, and cases of dengue fever are on the rise this year. Unfortunately, no vaccine is available in the U.S. to protect people against dengue, so be sure to use an insect repellant with at least 20% DEET."
"But, the good news for U.S. travelers is you can now vaccinate (IXCHIQ®) against the chikungunya illness and travelers no longer need to take malaria medication while in El Salvador because it has been eradicated from all parts of the country," added Beales, who leads Destination Health Clinic. This Boston-area travel health provider specializes in health education and vaccination for international travelers.
The CDC suggests visiting your healthcare provider at least a month before your trip to El Salvador to acquire necessary vaccines such as chikungunya or typhoid. Visit the CDC's page for the latest Travel Health Information.