Search API
The Committee on Immunization of Quebec (CIQ) today recommended using RSV (respiratory syncytial virus) vaccines, including AREXVY™, to prevent RSV among older adults at increased risk of severe outcomes from the virus.
Specifically, CIQ recommends vaccination for older adults living in residential and long-term care centers, intermediate resources, adults aged 75 and older living in private seniors’ residences, and those living in the community with chronic illnesses.
The CIQ recommendations for RSV vaccination follow those issued by the National Advisory Committee on Immunization, published earlier in July 2024.
Marni Freeman, Country Medical Director, GSK Canada, said in a press release on July 24, 2024, “As our immune system ages, we all become more vulnerable to severe consequences of RSV disease."
"Older adults who are immunocompromised or suffer from underlying medical conditions, such as chronic heart or lung disease, are at an even greater risk.
"The CIQ recommendation reflects the important role AREXVY can play in reducing the incidence and overall burden of respiratory syncytial virus among Quebec’s older adult population, and we look forward to collaborating with public health officials, healthcare professionals, and payers to ensure optimal vaccine access in the province.”
AREXVY was approved in Canada in August 2023 and is indicated for preventing lower respiratory tract disease caused by RSV in individuals 60 and older.
This vaccine (May 2023), two other RSV vaccines, and one monoclonal antibody for infants have been approved for use in the United States.
RSV is a common, contagious virus that affects the lungs and respiratory airways. For most people, the virus causes cold-like symptoms. Still, for older adults and adults with certain health conditions, it can lead to more serious infections and complications such as pneumonia, hospitalization, and even death, says the CIQ.

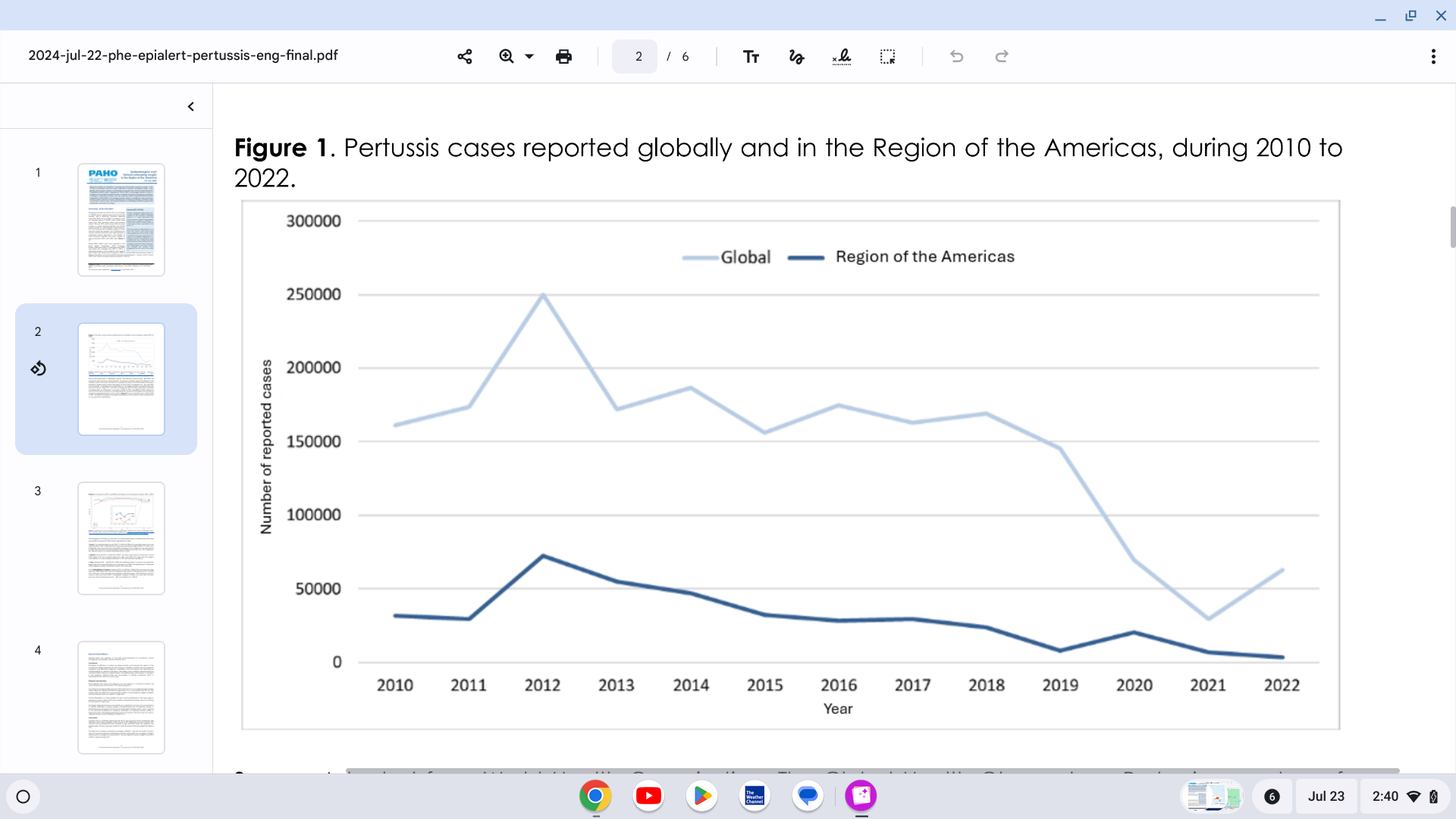
The Pan American Health Organization (PAHO) reported yesterday a significant increase in pertussis (whooping cough) cases in the Region of the Americas.
On July 22, 2024, the PAHO confirmed that 7,251 pertussis cases were reported in the United States in 2024, a 300% increase from last year.
Pertussis cases in Mexico are 242% higher than reported in 2023. Brazil and Peru are also reporting measurable case increases.
In the Region of the Americas, 2012 was the year with the highest number of cases reported during the decade, with 72,328 reported cases of pertussis. Since then, there has been a progressive annual decrease in the reported cases, reaching the lowest number reported in 2022, with 3,283 pertussis cases.
The first and third doses of diphtheria, tetanus, and pertussis vaccines (DTP1 and DTP3) are commonly used as tracers of immunization coverage. The coverage trend for both first and third doses has shown a significant decline.
The year 2021 was the lowest coverage year in the Region of the Americas compared with the previous 20 years. However, updated vaccine coverage data for 2023 reported a recovery of 90% for DTP1 and 88% for DTP3.
Globally, respiratory syncytial virus (RSV) is the leading cause of hospitalization for healthy infants under a year old and causes an estimated 101,000 deaths a year in children under five.
To address this significant health risk, Merck today announced positive topline results from its Phase 2b/3 clinical trial evaluating clesrovimab (MK-1654), the company’s investigational prophylactic monoclonal antibody (mAb) designed to protect infants from respiratory syncytial virus (RSV) disease.
Clesrovimab met its primary safety and efficacy endpoints in the trial, including reducing medically attended lower respiratory infections caused by RSV through Day 150.
Clesrovimab is being studied in infants (pre-term and full-term) to provide rapid, durable protection through their first RSV season with a single, fixed-dose administration.
“RSV is highly contagious and can cause inflammation in the airways of infants, leading to difficulty breathing. As a widespread illness globally, RSV is the leading cause of hospitalization for healthy infants,” said Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on July 23, 2024.
“We are encouraged by these findings and look forward to working with regulators to provide a new option to help address the impact of RSV on infants and their families."
For the 2024-2025 RSV season, the U.S. FDA-approved Beyfortus™ (Nirsevimab) mAb offers passive immunization to prevent lower respiratory tract infections caused by the RSV to infants experiencing their first or second RSV season and those with congenital heart disease or chronic lung disease.
Additionally, one vaccine has been approved for pregnant women, which offers RSV protection to newborns.

In the WHO Eastern Mediterranean Region, dengue outbreaks continue to be reported in 2024. The Ae. aegypti and some Ae. albopictus dengue virus-carrying mosquitoes have been identified in most of the countries in this region.
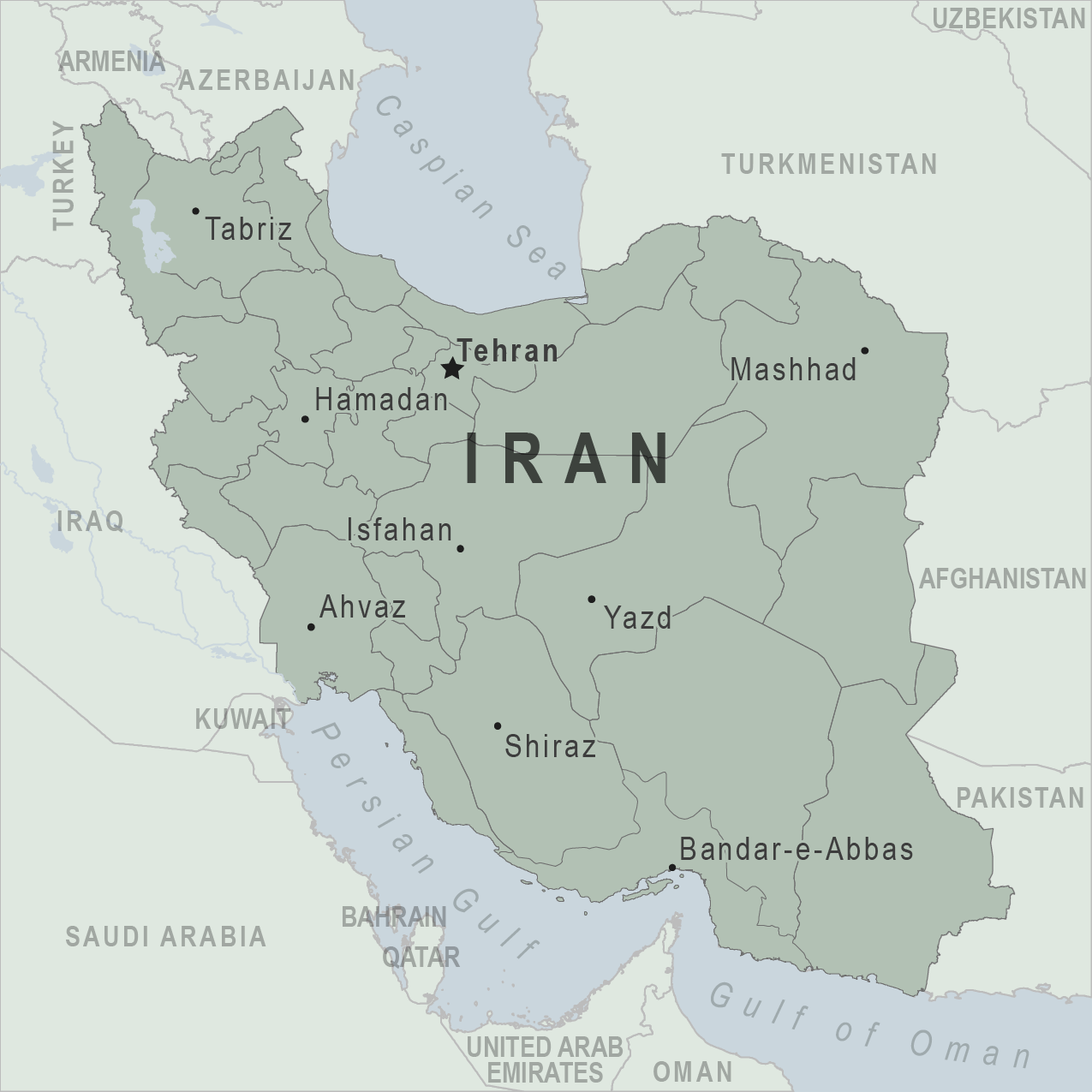
On July 17, 2024, the WHO Disease Outbreak News reported 12 autochthonous (local) cases of dengue documented in Iran, all of which were reported in Bandar-Lengheh, Hormozgan Province.
Additionally, there have been 137 travel-related dengue cases in 2024.
Iran reported an average of 20 imported dengue cases annually between 2017 and 2023.
Furthermore, WHO does not recommend any general travel or trade restrictions in Iran based on the available information.
The WHO recommends that vaccination against dengue be part of an integrated strategy to control the disease, including vector control, proper case management, community education, and community engagement.
WHO recommends that countries consider introducing the second-generation QDENGA® (TAK-003) vaccine into their routine immunization programs in locations where high transmission intensity of dengue poses a significant public health problem.
WHO does not currently recommend the programmatic use of TAK-003 in young children.
As of July 22, 2024, the QDENGA vaccine is not authorized for use in the United States.
The U.S. government recently exercised a procurement option to enhance orthopoxvirus preparedness against mpox and smallpox outbreaks.
On July 19, 2024, SIGA Technologies, Inc. announced that the U.S. Department of Health and Human Services (HHS) ordered the delivery of approximately $113 million of oral TPOXX® (tecovirimat) treatment courses.
“Building on the orders received in 2023 from the U.S. government and 15 international customers, this $113 million order from the U.S. government will enhance orthopoxvirus preparedness and support sizable and consistent action when needed to help ensure public health from natural, accidental, or intentional threats,” said Diem Nguyen, Chief Executive Officer, in a press release.
TPOXX is an antiviral medicine approved by the U.S. Food and Drug Administration (July 2018) specifically for treating smallpox disease in adults and pediatric patients weighing at least 13 kg.
Following U.S. approval, Health Canada also authorized TPOXX for the treatment of mpox and smallpox and authorized in Europe and the UK to treat smallpox, mpox, cowpox, and vaccinia complications.
As of July 22, 2024, mpox vaccines were also approved by the U.S. FDA and are available in the United States, Canada, and various countries.

With over 24,600 Zika virus cases already confirmed in the Region of the Americas in 2024, a new study published by the journal eBioMedicine, part of The Lancet, disclosed new, unsettling insights.
The Cleveland Clinic announced that this study revealed that maternal Zika virus infections can reprogram fetal immune development, leading to long-term consequences in children's immunity.
On July 18, 2024, the Clinic stated that these changes may occur in children born without the physical characteristics associated with congenital Zika syndrome, such as microcephaly.
This finding suggests that 95% of babies born from Zika-infected pregnancies who did not exhibit symptoms may have been affected by the virus with long-term immunological repercussions.
Additionally, heightened inflammation was observed in Zika-exposed infants with abnormalities at birth, while children exposed to Zika later maintained a chronic Th1-biased immune profile. The impaired response to Th2-biased vaccines raises concerns about the lasting effects of Zika virus exposure on immune responses.
Suan-Sin (Jolin) Foo, PhD, an expert in maternal-fetal virology and the Zika virus, says babies without symptoms are deemed healthy and do not receive any follow-up medical care or attention.
“Studies have only really focused on what’s happening with the children who were born with visible physical conditions like microcephaly or neurological complications,” Dr. Foo commented in a press release.
“The rest of these kids may not even have a note on their chart mentioning that their mother was infected during pregnancy. Unless they’re part of our study, they’re essentially lost to the medical field.”
Dr. Foo added, “Our study clearly shows there’s much more to this condition than meets the eye. We need to expand diagnostic criteria and conduct more research to ensure these immunologically vulnerable children get the necessary care.”
From a prevention perspective, no Zika vaccine candidate has been approved by the U.S. FDA as of July 2024.

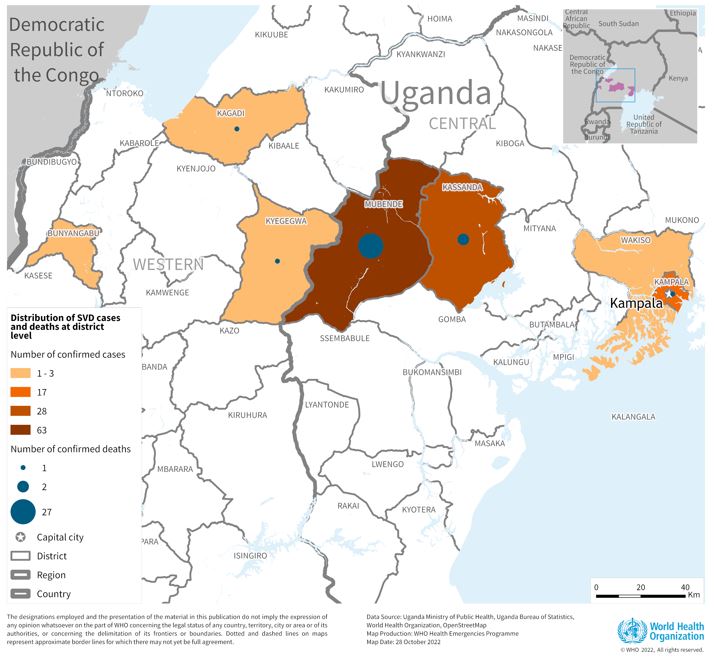
The Sabin Vaccine Institute recently announced the launch of a Phase 2 clinical trial for its vaccine against Sudan ebolavirus at Makerere University Walter Reed Project (MUWRP) in Uganda.
This is a vital development as there are currently no approved vaccines for this strain of ebolavirus.
Based on the cAd3 platform, Sabin’s single-dose investigational Sudan ebolavirus vaccine was found to be promising in Phase 1 clinical and non-clinical studies. Results showed it to be safe while eliciting rapid and robust immune responses that lasted up to 12 months.
“We are delighted to advance a vaccine candidate that can thwart a deadly and devastating disease, especially one that caused a fairly recent outbreak and for which no approved treatments exist,” commented Amy Finan, Sabin’s Chief Executive Officer, in a press release on July 15, 2024.
“Sabin’s vaccine candidate is backed by strong safety and immunogenicity data, and we hope this trial will yield further evidence to move the vaccine closer to licensure.”
This is Sabin’s second Phase 2 clinical trial partnership with MUWRP, based in Uganda’s capital, Kampala. A Phase 2 trial for a Marburg vaccine is already underway, having recently completed enrollment. Initial results from the Marburg trial are expected later this year.
The most recent outbreak of Sudan ebolavirus occurred in Uganda in the fall of 2022. That outbreak ultimately resulted in 55 deaths.
Sabin’s vaccine candidate was the first to arrive in Uganda during that outbreak after the WHO included it as one of three vaccines for possible use in an outbreak trial. The outbreak ended before the vaccine was deployed.
In August 2019, Sabin announced agreements with GSK to advance the development of vaccines against the Zaire and Sudan ebolavirus and Marburg virus. The three candidate vaccines were initially developed collaboratively by the U.S. National Institutes of Health and Okairos, acquired by GSK in 2013.
As of July 20, 2024, the U.S. FDA has approved Zaire Ebolavirus vaccines, which have been offered in Africa since 2019.