Search API
According to a recent article published by npj vaccines, infection with high-risk human papillomavirus (HPV) is widely recognized as the primary cause of cervical and other malignant cancers.
While there are six licensed prophylactic vaccines against HPV, none of them show any significant therapeutic effect on pre-existing infections.
Thus, a prophylactic vaccine endowed with therapeutic activity would afford protection regardless of the vaccine recipient's HPV infection status.
The key to eliminating virus-infected cells lies in anti-early HPV-encoded proteins, particularly anti-E6 or anti-E7 T-cell responses.
This article describes the refinement and further potentiation of a dual-purpose HPV nanoparticle vaccine (cPANHPVAX) relying on eight different HPV L2 peptide epitopes and the E7 oncoantigens from HPV16 and 18.
cPANHPVAX not only induces anti-HPV16 E7 cytotoxic T-cell responses in C57BL/6 mice, but also anti-HPV18 E7 T-cell responses in transgenic mice with the A2.DR1 haplotype.
These cytotoxic responses add to a potent, broad-coverage humoral (HPV-neutralizing) response.
cPANHPVAX safety was further improved by deleting the pRb-binding domains of E7.
These researchers wrote, 'this dual-purpose vaccine holds great potential for clinical translation as an immune-treatment capable of targeting active infections as well as established HPV-related malignancies, thus benefiting both uninfected and infected individuals.'
Zhao, X., Zhang, Y., Trejo-Cerro, O. et al. A safe and potentiated multi-type HPV L2-E7 nanoparticle vaccine with combined prophylactic and therapeutic activity. npj Vaccines 9, 119 (2024). https://doi.org/10.1038/s41541-024-00914-z.

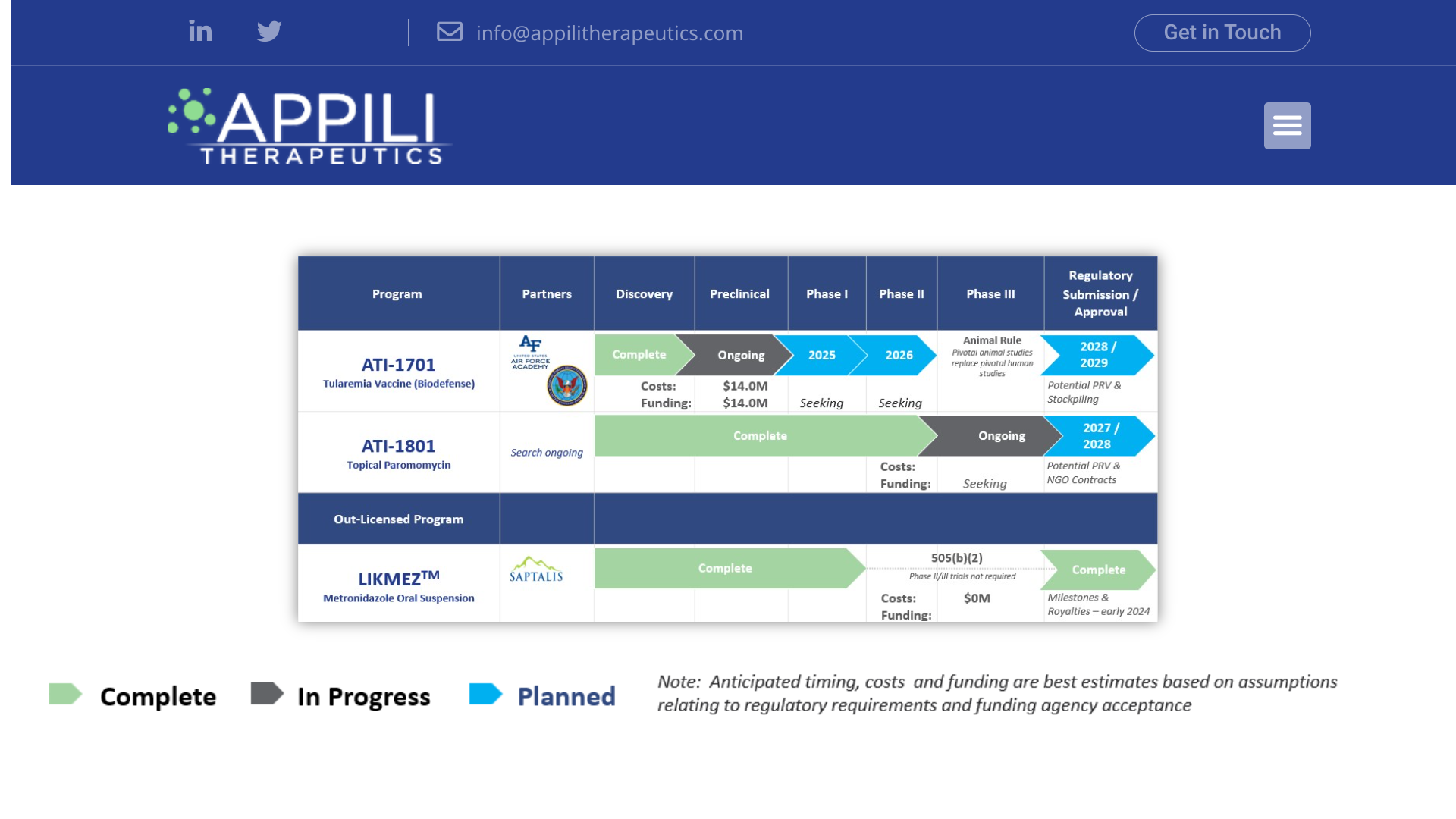
Appili Therapeutics Inc. recently announced a product update for the first quarter of its fiscal year 2025, which ended on June 30, 2024.
“In recent months, we’ve achieved significant milestones by engaging regulatory authorities for ATI-1701 and ATI-1801, and together with our partner, Saptalis Pharmaceuticals, we launched our leading asset, LIKMEZ™ (ATI-1501),” said Don Cilla, President and CEO of Appili, in a press release on August 13, 2024.
“These achievements, together with ATI-1701 U.S. Air Force Academy (USAFA) funding commitments, are expected to enable us to execute our infectious disease programs and advance their development to bring these products to market for the benefit of patients and shareholders.”
ATI-1701, a novel live-attenuated vaccine for preventing F. tularensis, has secured $14 million in awards from the USAFA. Tularemia is a potentially serious illness caused by the bacterium Francisella tularensis. People can become infected in several ways, including tick and deer fly bites and contact with infected animals.
Under the USAFA Cooperative Agreement, Appili will manage a comprehensive development program, including nonclinical studies, CMC/manufacturing, clinical preparatory, and regulatory activities supporting an IND submission in 2025. Appili has engaged with the U.S. Food and Drug Administration (“FDA”) via a pre-IND meeting, confirming the development pathway for ATI-1701, and is incorporating suggested changes into the development plan.
ATI-1801, a novel topical formulation of paromomycin (15% w/w), is under advanced clinical development for treating cutaneous leishmaniasis, a Neglected Tropical Disease, is a disfiguring skin infection affecting hundreds of thousands globally. Cutaneous and mucosal leishmaniasis can cause substantial morbidity; visceral and mucosal leishmaniasis can be life-threatening.
Appili is currently engaging with the FDA. In 2024, it submitted a type-B meeting request to discuss linking previously generated Phase 3 data and agreeing on the necessary registration package for a New Drug Application submission. ATI-1801 has received an Orphan Drug Designation from the FDA for certain forms of cutaneous leishmaniasis.
In September 2023, Appili and its U.S. partner, Saptalis Pharmaceuticals LLC., announced the approval by the U.S. FDA of LIKMEZ™ (ATI-1501), a proprietary taste-masked liquid suspension formulation of metronidazole. LIKMEZ is the first FDA-approved ready-made suspension of metronidazole, addressing the unmet need in both pediatric patients and patients with dysphagia. Saptalis launched LIKMEZ in November 2023, and the product is available to patients and doctors in the U.S.
Eisai Co., Ltd. and Biogen Inc. announced that the Ministry of Health and Prevention in the United Arab Emirates (UAE) has approved humanized anti-soluble aggregated amyloid-beta monoclonal antibody LEQEMBI® (lecanemab) for the treatment of Alzheimer’s disease (AD).
LEQEMBI is the first approved treatment shown to reduce the rate of disease progression and to slow cognitive and functional decline through this mechanism.
In the UAE, AD is considered the most common cause of dementia, typically accounting for 60-70% of cases. It is reported that 4.09% of people over 60 years old have dementia.
As of August 13, 2024, LEQEMBI is also approved in the U.S., Japan, China, South Korea, Hong Kong, and Israel and is being marketed in the U.S., Japan, and China.
Eisai has also submitted applications for approval of LEQEMBI in 11 countries and regions.
In the United States, a supplemental Biologics License Application for intravenous maintenance dosing was submitted to the U.S. FDA in March 2024 and was accepted in June 2024. The rolling submission of a Biologics License Application for maintenance dosing of a subcutaneous injection formulation, which is being developed to enhance patient convenience, was initiated in the U.S. under FDA Fast Track status in May 2024.
Since the beginning of 2022 and as of late July 2024, a total of 37,583 mpox cases and 1,451 deaths (case fatality rate 3.9%) have been reported from 15 African Union Member States. In an attempt to reduce this outbreak, the U.S. FDA and EMA-approved mpox vaccine is being deployed.
Bavarian Nordic A/S recently announced a new order from the European Health Emergency Preparedness and Response Authority (HERA) for the MVA-BN® (JYNNEOS) vaccine.
HERA will procure 175,420 vaccine doses to donate to the Africa Centres for Disease Control and Prevention (ACDC) to support their strengthened response to the ongoing mpox outbreak in Africa.
Additionally, Bavarian Nordic will donate 40,000 doses to HERA, which will also be donated to the ACDC.
This larger donation follows a recent pledge from Bavarian Nordic for 15,000 doses as part of a coordinated response by Gavi, WHO, and UNICEF in the African region.
“Mpox is spreading at an alarming rate in Africa, calling for further action from the international community. We are proud to support HERA’s contribution of vaccines to the region and are pleased to announce an additional donation from Bavarian Nordic,” said Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, in a press release on August 13, 2024.
Currently, two African countries have granted Emergency Use Authorization for the MVA-BN vaccine.
Seperately, the WHO Director-General issued the following statement on August 14, 2024, "In light of the expanding outbreak in east and central Africa, and the potential for further international spread within and outside Africa, I have convened this Emergency Committee under the International Health Regulations to advise me on whether the outbreak represents a public health emergency of international concern."
"When I declared an end to the previous mpox PHEIC last year (2023), I issued standing recommendations under the IHR, which are due to expire next week. I have decided to extend them for another year to support countries in responding to the chronic risk of mpox."
"Were I to decide, on your advice, that the current situation represents a public health emergency of international concern, I would issue temporary recommendations by the IHR, again on your advice."
The JYNNEOS vaccine is available in the United States at designated clinics and pharmacies.
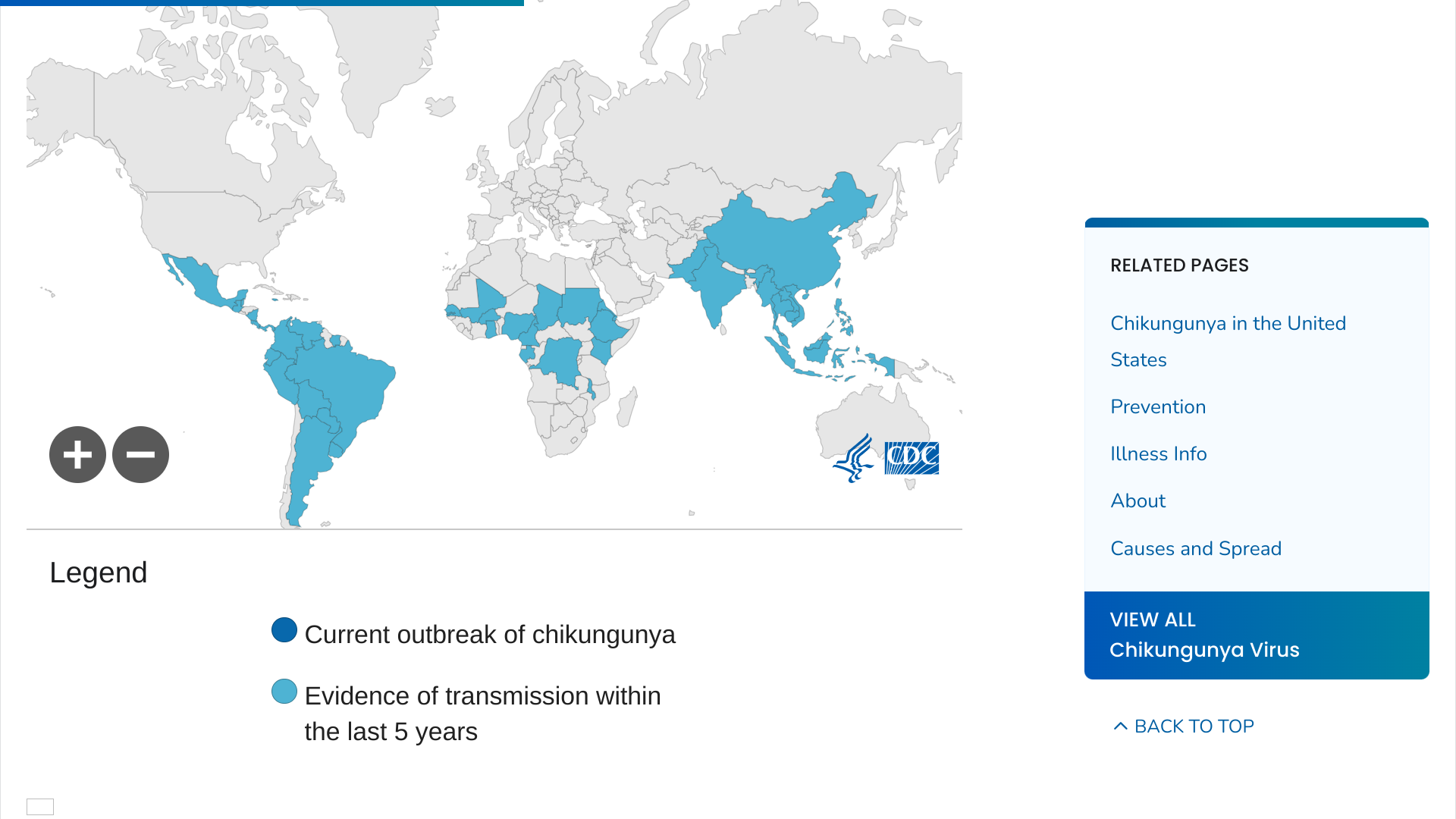
With one approved chikungunya vaccine already available, the U.S. Food and Drug Administration (FDA) has accepted and granted Priority Review for the Biologics License Application (BLA) for Bavarian Nordic A/S CHIKV VLP, a vaccine candidate for immunization to prevent disease outbreaks caused by chikungunya virus infection in individuals 12 years of age and older.
The Priority Review designation means the FDA aims to complete its review within six months. The FDA has assigned a target action date for the Prescription Drug User Free Act of February 14, 2025.
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization to prevent disease caused by CHIKV infection.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release on August 13, 2024, “The FDA review, along with the ongoing review of our CHIKV VLP vaccine by the European Medicines Agency, represent the first regulatory reviews of a chikungunya vaccine for adolescents, potentially providing a broader usage by populations at risk of this debilitating disease.”
CHIKV VLP is currently also under accelerated assessment review with the EMA, potentially supporting approval of the vaccine by the European Commission in the first half of 2025.
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus (CHIKV). The disease typically presents with acute symptoms, including fever, rash, fatigue, headache, and often severe and incapacitating joint pain.
While mortality is relatively low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age.
Over the past few decades, CHIKV has emerged in several previously non-endemic regions in Asia, Africa, southern Europe, and the Region of the Americas, often causing large, unpredictable outbreaks.
As of August 8, 2024, the Pan American Health Organization (PAHO) reported over 371,167 CHIKV cases in the Americas this year. Between 2013 and 2023, the PAHO reported more than 3.7 million CHIKV cases in the Americas.
The U.S. CDC reported from 2006 to 2023, 4,590 travel-related CHIKV cases were reported in the U.S., in areas such as Florida.
However, Locally acquired cases have not been reported in U.S. states or territories since 2019.
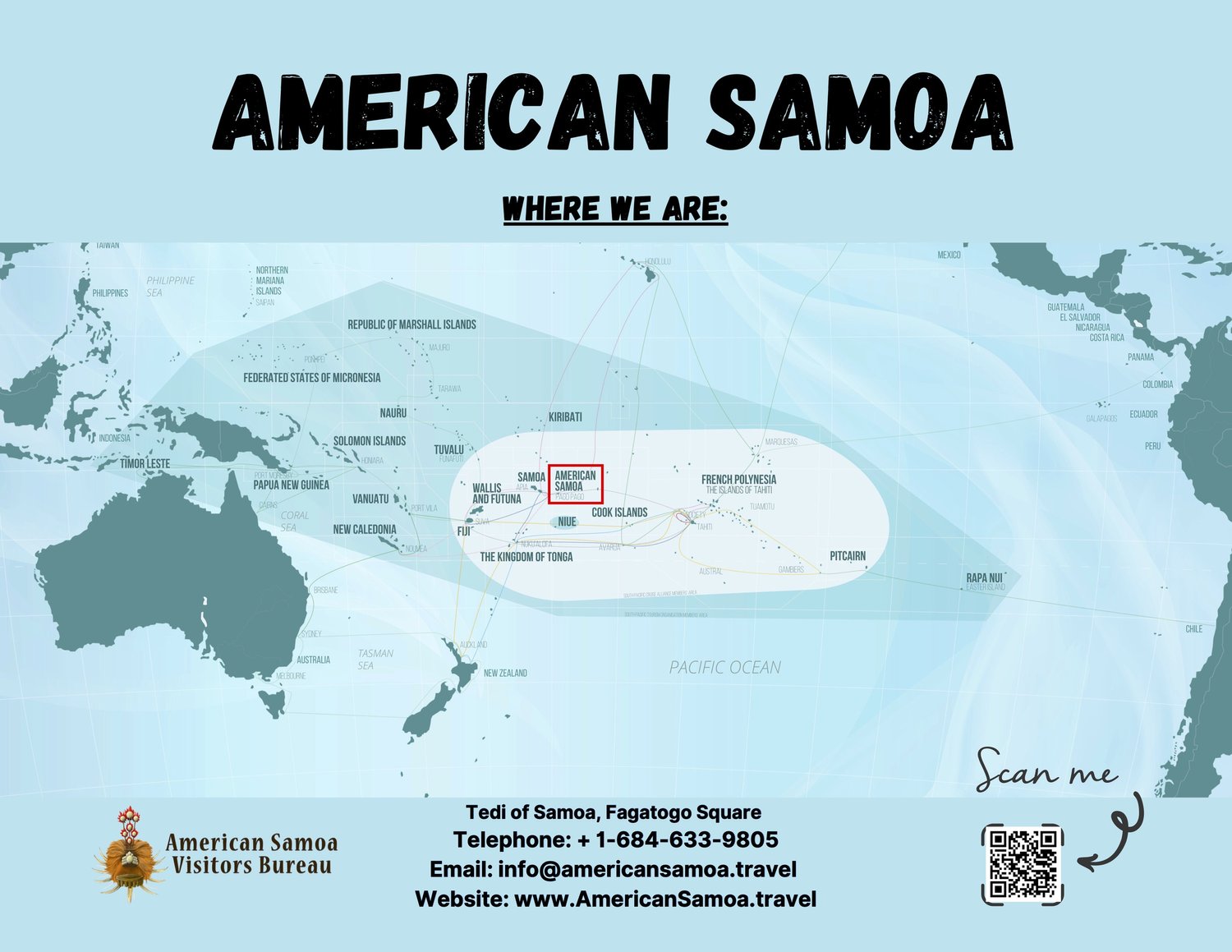
According to a recent Morbidity and Mortality Weekly Report, more than half of school-aged children in American Samoa have evidence of a dengue fever virus infection.
The estimated seroprevalence among all students aged 7–16 years was 59% (95% CI = 47%–71%) and was 60% (95% CI = 48%–72%) among those age-eligible for vaccination (i.e., those aged 9–16 years).
Dengue seroprevalence was lowest among children aged 8 (46%; 95% CI = 32%–60%).
The U.S. CDC's Notes from the Field (73(31);686–688) published on August 8, 2024, suggests American Samoa exceeds the minimum 20% threshold established for the introduction of recommended dengue vaccines to reduce the risk of hospitalization and severe dengue in seronegative children and adolescents.
The authors concluded, "In American Samoa, dengue vaccines could be part of a broader strategy for dengue control."
As of August 13, 2024, access to dengue vaccines in the United States is very limited.
American Samoa is located in the Pacific Ocean, halfway between Hawaii in the north and New Zealand in the south. The CDC recommends checking the vaccines and medicines list and visiting your healthcare provider at least a month before your trip to American Samoa to get necessary supplies.
These authors disclosed no potential conflicts of interest.