Search API
Without a herpes preventive vaccine available in 2024, post-infection treatments offer patients their best option.
Over the past few years, an antiviral drug named Acyclovir has been used to slow the spreading and lessen the symptoms of the Herpes Simplex Virus 1 (HSV-1) virus.
However, Acyclovir will not cure herpes.
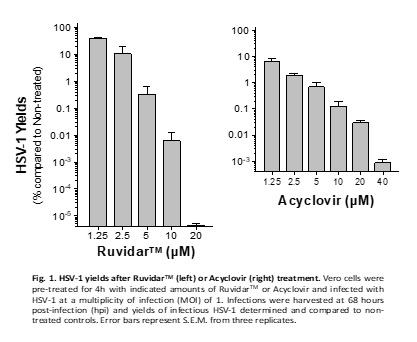
To address this need, Theralase® Technologies Inc. today announced that its lead drug formulation, Ruvidar™, could be more effective in destroying HSV-1 than Acyclovir.
Another important pre-clinical observation is that Acyclovir could not prevent HSV-1 replication if added one day after infection. However, Ruvidar prevented HSV-1 replication by ten million-fold when added one day after infection.
In other words, from a clinical perspective, if a patient has pre-existing HSV-1, then Acyclovir could not prevent the virus' replication; however, Ruvidar TM would be highly effective, the company wrote on September 3, 2024.
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase, stated in a press release, “This latest research continues to strengthen what we already know; Ruvidar is a very potent drug in the destruction of cancer, viruses, and bacteria on its own and is further enhanced by light, radiation, sound or drug activation."
"Based on this latest research, Theralase® plans to commence seeking a partner/licensing opportunity in the development of Ruvidar™ for both a topical and oral treatment for the prevention and treatment of herpes simplex.“
In previous research, Ruvidar was found effective at inactivating both enveloped and non-enveloped viruses.
Vaxcyte, Inc. today announced that it has commenced an underwritten public offering of $1.0 billion of its common stock and pre-funded warrants.
As of August 3, 2024, Vaxcyte intends to grant the funding underwriters a 30-day option to purchase up to an additional $150 million of shares of its common stock offered in the public offering (including shares underlying the pre-funded warrants).
Vaxcyrte confirmed this offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed or as to the actual size or terms of the offering.
The Company is developing broad-spectrum conjugate and novel protein vaccines to prevent or treat bacterial infectious diseases.
VAX-31 is a Phase 3-ready 31-valent, carrier-sparing pneumococcal conjugate vaccine (PCV) candidate being developed for the prevention of invasive pneumococcal disease (IPD) in adults and infants and is the broadest-spectrum PCV candidate in the clinic today.
VAX-24, the Company’s 24-valent PCV candidate, is designed to cover more serotypes than any infant PCV on-market and is currently being evaluated in a Phase 2 infant study.
Both VAX-31 and VAX-24 are designed to improve upon the standard-of-care PCVs by covering the serotypes in circulation that are responsible for a significant portion of IPD and are associated with high case-fatality rates, antibiotic resistance, and meningitis while maintaining coverage of previously circulating strains that are currently contained through continued vaccination practice.

Vaxcyte, Inc. today announced positive topline results from the Phase 1/2 study evaluating the safety, tolerability, and immunogenicity of VAX-31, the Company’s 31-valent pneumococcal conjugate vaccine (PCV) candidate designed to prevent invasive pneumococcal disease (IPD).
In this Phase 1/2 study, VAX-31 was observed to be well tolerated and demonstrated a safety profile at all doses studied through the full six-month evaluation period similar to Prevnar 20® (PCV20).
The VAX-31 vaccine candidate showed robust opsonophagocytic activity (OPA) immune responses for all 31 serotypes at all doses studied.
At the middle and high doses, VAX-31 met or exceeded the OPA response non-inferiority criteria for all 20 serotypes common with PCV20.
At the VAX-31 high dose, average OPA immune responses were greater for 18 of 20 serotypes compared to PCV20 (geometric mean ratio (GMR) greater than 1.0), with seven of these serotypes achieving statistically higher immune responses compared to PCV20.
At the middle dose, 13 of 20 serotypes had a GMR greater than 1.0, and five serotypes achieved statistically higher immune responses compared to PCV20. For all 11 incremental serotypes unique to VAX-31 and not in PCV20, all three doses met the superiority criteria.
Based on the strength of the results from this study, the Company has selected VAX-31 to advance to an adult Phase 3 program. The Company plans to select the VAX-31 dose before initiating the adult Phase 3 program.
Grant Pickering, Chief Executive Officer and Co-Founder of Vaxcyte commented in a press release on September 3, 2024, “Based on the strength and clarity of these data, we have selected VAX-31 for the adult indication and plan to initiate the pivotal, non-inferiority Phase 3 study by mid-2025 and announce topline data in 2026."
"We intend to initiate the remaining VAX-31 Phase 3 studies in 2025 and 2026 and submit a Biologics License Application subject to the results of these studies.”
Pneumococcal disease is an infection caused by Streptococcus pneumoniae bacteria, leading to thousands of hospitalizations yearly in the U.S.

Valneva SE and Pfizer Inc. today announced positive immunogenicity and safety data from their VLA15-221 Phase 2 study following a second booster vaccination of their Lyme disease vaccine candidate, VLA15, given one year after receiving the first booster dose.
VLA15 is an investigational multivalent protein subunit vaccine that uses an established mechanism of action for a Lyme disease vaccine. It targets the outer surface protein A of Borrelia burgdorferi, the bacteria that causes Lyme disease.
There are currently no approved human vaccines for Lyme disease, and VLA15 is the candidate that has advanced the furthest along the clinical development timeline, with two Phase 3 trials in progress.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a September 3, 2024, press release, "We are encouraged by these data, which support the potential benefit of booster doses across all examined age groups."
"As Lyme disease continues to spread, it represents a significant unmet medical need, affecting numerous individuals throughout the Northern Hemisphere."
"Each new set of positive data brings us one step closer to potentially bringing this vaccine to adults and children living in areas where Lyme disease is endemic."
Subject to positive Phase 3 data, Pfizer aims to submit a Biologics License Application to the U.S. FDA and a Marketing Authorization Application to the European Medicines Agency in 2026.
The Centers for Disease Control and Prevention (CDC) estimated that approximately 476,000 people in the U.S. are diagnosed and treated for Lyme disease yearly. This disease was first identified in Lyme, Connecticut.
On August 14, 2024, the CDC reported (Volume 30, Number 9—September 2024) that the overall incidence of Lyme disease was about seven times higher than that reported through public health surveillance.
Unfortunately, the northeastern United States is the leading area for Lyme disease-carrying ticks.
For example, the Pennslyvania Department of Health Tickborne Disease Dashboard shows that while cases of Lyme disease peak in June through August, the threat exists year-round, primarily in the northwestern section of the state.

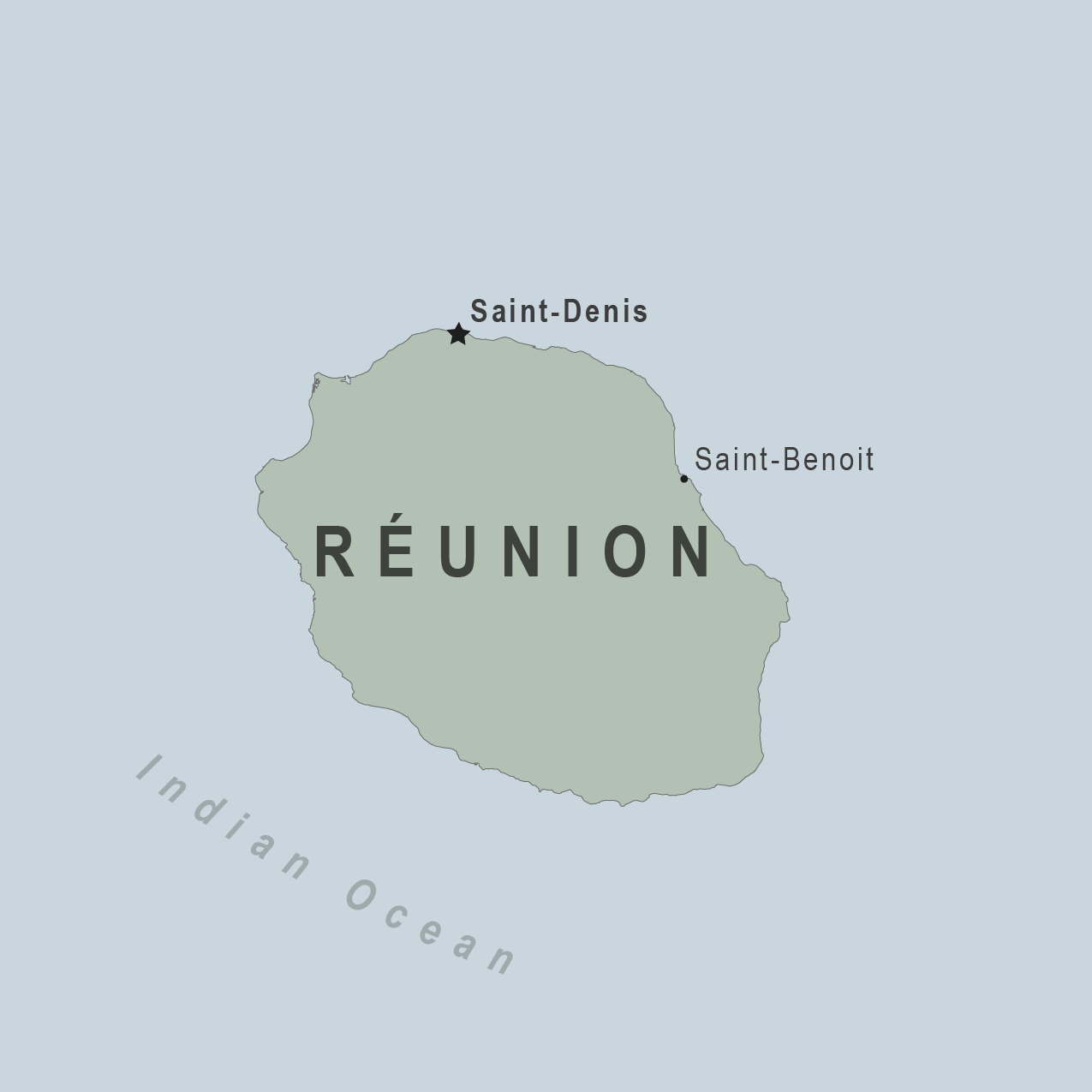
The European CDC Communicable Disease Threats Report, published on August 30, 2024, disclosed that France had reported three autochthonous (locally-acquired) chikungunya virus disease cases in the Department of La Réunion from the same neighborhood.
The last major chikungunya outbreak in La Réunion was from 2005 to 2006.
The ECDC says the risk of chikungunya infection for residents and travelers to La Réunion is currently low. This mosquito-transmitted disease activity has been reduced as it's winter in La Réunion.
However, further cases cannot be excluded, says the ECDC.
La Réunion is an island in the Indian Ocean east of Madagascar and Africa that welcomes over 350,000 visitors annually.
As of September 2, 2024, the U.S. CDC had not issued a Travel Healht Advisory regarding La Réunion's chikungunya outbreak.
From a disease prevention option, Valneva SE's IXCHIQ® single-dose, live-attenuated chikungunya vaccine has been approved by the U.S. FDA and throughout Europe. Travel vaccination services are offered throughout the United States at certain pharmacies and Passport Health USA.
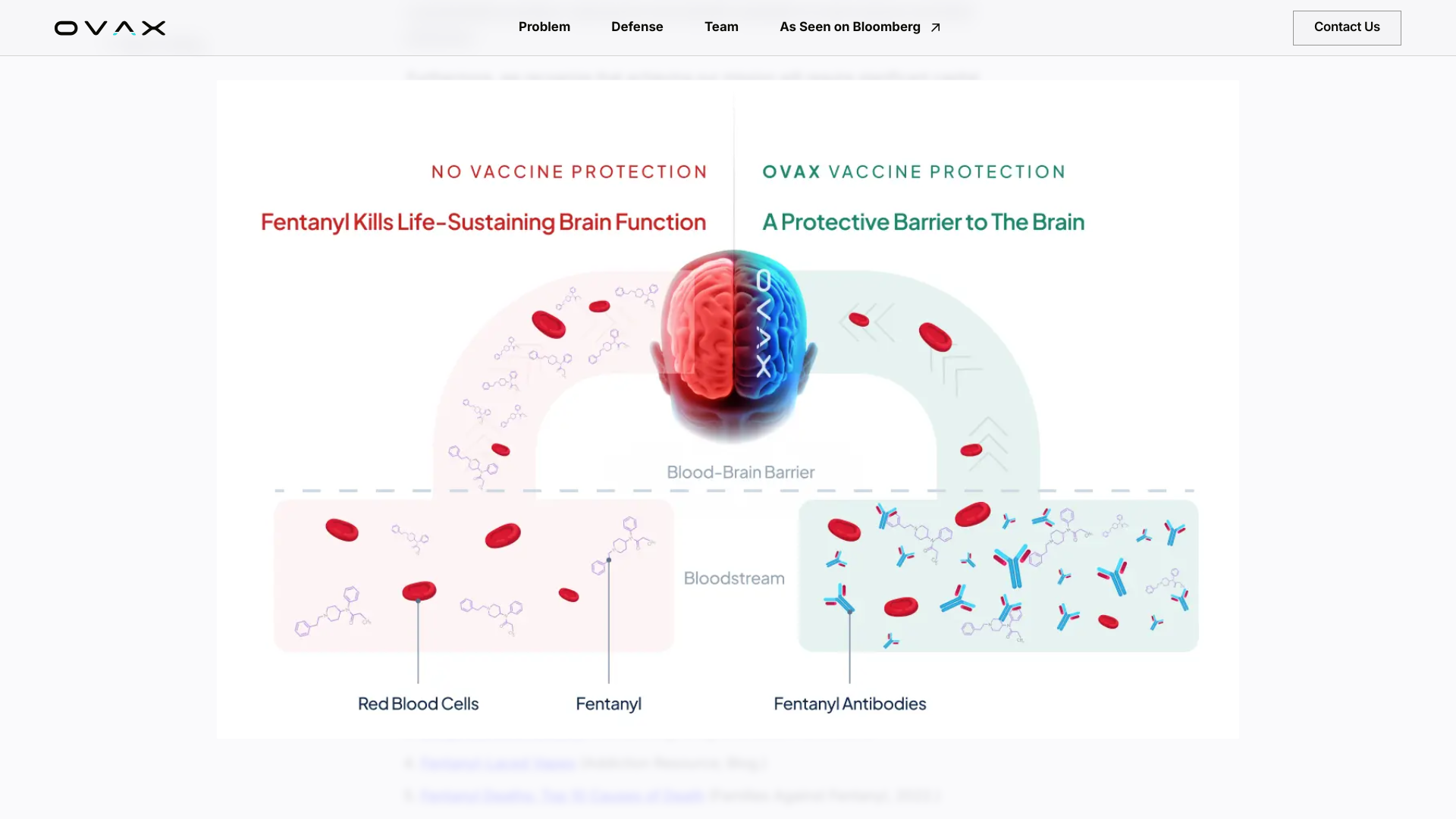
OVAX Inc. is now emerging from stealth mode with an initial $10 million seed funding backed by its team, concerned parents, and mission-aligned investors. According to Pitchbook reporting, no enterprise valuation was disclosed.
OVAX is developing Fentanyl Armour nasal spray vaccine technology designed to stop fentanyl overdoses. Illicit fentanyl kills approximately 200 Americans daily.
Fentanyl is a synthetic opioid that is often added to illegal drugs, says the U.S. CDC.
The company offers a defense system that immediately neutralizes fentanyl upon exposure and blocks the euphoric effects, enabling the healthcare industry to prevent overdoses leading to death.
On June 24, 2024, the company's media statement confirmed, 'Our vaccines (candidates) are designed to be taken a few times per year with several options in development, including a convenient nasal spray designed to be administered in the comfort of one's home, eliminating the requirement for a needle injection.'
The company licensed intellectual property from the University of Houston, Texas, which completed pre-clinical evaluations.
According to Ovax executives' comments, they plan to launch human clinical trials of Fentanyl Armour in 2025.
'We recognize that achieving our mission will require significant capital investment and a dedicated team. Additionally, OVAX will work alongside key collaborators at non-profits, governments, and communities dedicated to solving the fentanyl crisis,' wrote the company.
As of September 2024, there are no approved vaccines targeting fentanyl overdosing.
A new study from researchers at Wake Forest University School of Medicine sheds light on how the U.S. news media recently portrayed scientific evidence and the uncertainty surrounding unproven therapeutics.
The research team analyzed news reports on how scientific evidence, evidence details and limitations, safety, efficacy, and sources of authority were portrayed to the public.
“We found that 67% of news reports included scientific evidence, but only 24% mentioned scientific publications or journals,” said the study’s corresponding author in a press release on August 29, 2024.
Zubin Master, Ph. D., associate professor of social sciences and health policy at Wake Forest University School of Medicine, commented, “This period of time (the recent pandemic) was when medical specialists and the general public were anxiously scrambling to learn as much as possible about prevention and treatments because there were yet no proven therapeutics or vaccines."
"This makes for an ideal case study to examine how the news media portrays scientific evidence.”
According to the American Press Institute, only 40% of the public read news articles beyond headlines or lead paragraphs.
“It’s crucial, especially with controversial science topics, that the evidence and uncertainty are featured more prominently,” Master said.
The study authors also noted that science can be strengthened by acknowledging limitations and by portraying science as a process that is constantly changing and being corrected as additional knowledge is gained.
These findings appear online in the Journal of Medical Internet Research Infodemiology.