Search API
Bavarian Nordic A/S and Gavi today announced an advance purchase agreement (APA) to secure 500,000 doses of the MVA-BN® mpox vaccine (JYNNEOS® or IMVANEX®).
These vaccines are being supplied to African countries impacted by the current mpox clade 1 outbreak.
Bavarian Nordic is ready to supply the mpox/smallpox vaccines pending signing a supply agreement with UNICEF, Gavi’s alliance partner, which will deliver these doses in 2024.
Paul Chaplin, President & CEO of Bavarian Nordic, commented in a press release on September 18, 2024, “The doses secured through this APA will significantly increase the availability of mpox vaccines for African countries, and we are pleased that Gavi has selected our MVA-BN vaccine, which has proven highly effective during the global mpox (clade 2) outbreak in 2022.”
The vaccines will be funded by Gavi, the Vaccine Alliance's First Response Fund, a new financial mechanism created in June 2024 to make cash available to purchase vaccines in health emergencies rapidly.
As of September 2024, there are four mpox vaccines available globally.

Based on today's announcement by Valneva SE, adolescents may soon have access to the only approved chikungunya vaccine. This is essential news as the chikungunya virus (CHIKV) has now been identified in over 110 countries in Asia, Africa, Europe, and the Americas.
On September 18, 2024, Valneva submitted label extension applications to the European Medicines Agency (EMA) and Health Canada to potentially expand the use of its approved chikungunya vaccine, IXCHIQ®, to adolescents aged 12 to 17 years in Europe and Canada.
The Canadian label extension application also includes two-year antibody persistence data, a key differentiator for IXCHIQ® that was already included in the initial EMA filing.
Valneva expects to submit data to the U.S. Food and Drug Administration (FDA) in 2024 to support potential label extensions in the U.S.
IXCHIQ is currently approved in the U.S., Europe, and Canada to prevent disease caused by mosquito-spreading CHIKV in individuals 18 and older.
In the U.S., the commercial launch is underway, as IXCHIQ is available at health clinics and pharmacies.
First sales in Canada and Europe are anticipated in the fourth quarter of 2024.
In addition to ramping up sales, Valneva is focused on expanding the vaccine’s label and access.
The Company expects a marketing authorization in Brazil in the second half of 2024 and recently expanded its partnership with The Coalition for Epidemic Preparedness Innovations (CEPI) to support broader access to the vaccine in Low Middle-Income Countries, post-marketing trials, and potential label extensions in children, adolescents, and pregnant women.
CEPI previously confirmed it will provide Valneva up to $41.3 million of additional funding over the next five years, with support from the European Union’s Horizon Europe program.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, “Given the substantial risk that chikungunya presents to individuals residing in or traveling to endemic regions, it’s imperative to ensure the vaccine is available to all age groups."
"This broader accessibility would certainly help provide protection and mitigate the burden of this debilitating illness, which is currently spreading in areas that were previously unaffected."
"The durability of the immune response is also extremely important, especially for endemic countries where access to immunization can be difficult.”
EMA and Health Canada’s label extension applications are based on positive six-month adolescent Phase 3 data the Company reported in May 2024. These data showed that a single-dose vaccination with IXCHIQ® induces a high and sustained immune response in 99.1% of adolescents and that the vaccine was generally well tolerated.
The Lancet Infectious Diseases also recently published an article showing that the vaccine was well tolerated in adolescents aged 12 to 17 28 days after a single injection, regardless of previous CHIKV infection.
In addition to the adolescent data, Health Canada’s label extension application included IXCHIQ®‘s antibody persistence data, which showed that 97% of participants sustained the vaccine’s immune response for 24 months and was equally durable in younger and older adults.
Valneva expects to publish 36 months of persistence data later in 2024.

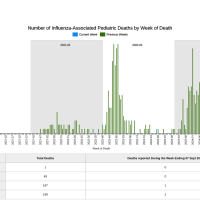
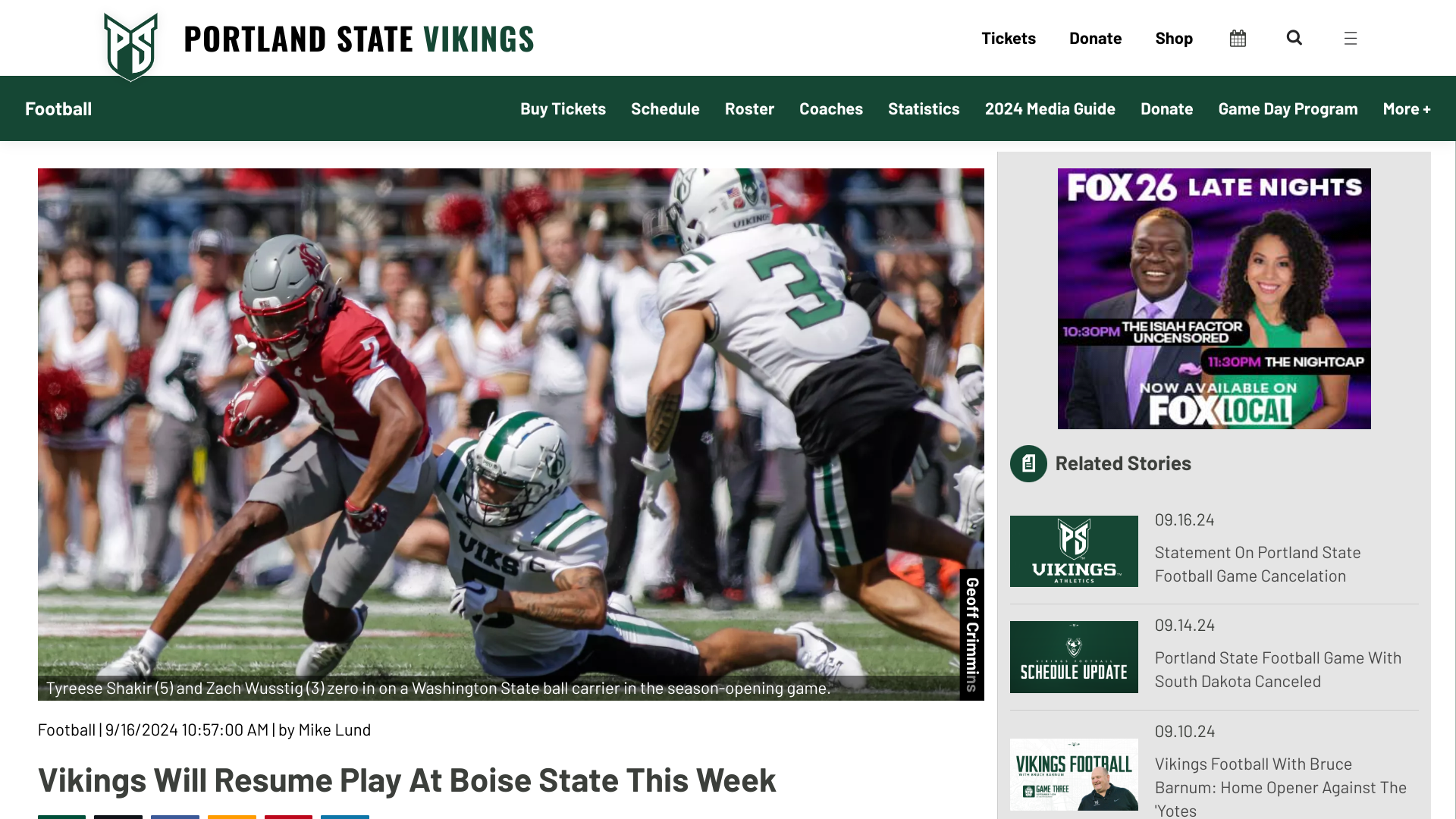
The Portland State University (PSU) and the South Dakota football game on September 14, 2024, was canceled due to a pertussis (whopping cough) outbreak.
Portland State announced a case of pertussis got into the Viking team two days before game day. As a result, many team members have been exposed to the highly contagious disease that affects the lungs. As a precaution, all PSU players exposed to the virus have begun a five-day course of antibiotics.
Portland State determined that the game in Oregon would not be played in the interest of student-athletes' health.
PSU team physicians Dr. Melissa Novak and Dr. Jacqueline Brady, employed by Oregon Health & Science University Sports Medicine, collaborate closely with the Multnomah County Health Department and the Oregon Health Authority to ensure the safety of the PSU community and the teams and universities. PSU will compete against them in the future.
The Vikings are scheduled to play at Boise State on September 21, 2024.
In 2024, reported cases of pertussis increased across the United States. Preliminary data published by the U.S. CDC show that more than four times as many cases have been reported as of August 17, 2024, compared to the same time in 2023, and higher than in 2019.
The CDC says vaccination is the best defense to prevent pertussis, a vaccine-preventable disease. Various vaccines are offered at health clinics and pharmacies in the U.S.
According to an analysis from the U.S. Centers for Disease Control and Prevention (CDC) and published by NEJM Evidence on September 13, 2024, SIGA Technologies, Inc. mpox treatment tecovirimat (TPOXX®) safety and effectiveness against the monkeypox virus clade 2 virus can't be determined from data.
CDC researchers evaluated data from over 7,100 patients prescribed tecovirimat, a virostatic antiviral drug, from May 29, 2022, through July 10, 2023.
They wrote, 'Although relatively few serious adverse events (SAEs) were reported, because of the passive nature of reporting, we cannot definitively conclude that tecovirimat treatment was always safe. Similar to data from case reports and other published observational studies, our data, in the absence of comparison data from untreated patients, cannot be used to infer clinical effectiveness, or lack thereof, of tecovirimat treatment.'
Overall, 223 SAEs and 40 deaths were reported. Most events were among patients who were severely immunocompromised.
Despite the inclusion of many patients with severe disease for whom the CDC was consulted, outcomes were favorable for most of the treated patients in this cohort.
This analysis did not review the current mpox clade 1 outbreak impacting countries in Africa.

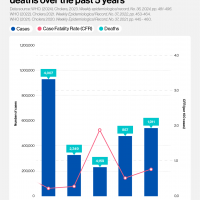
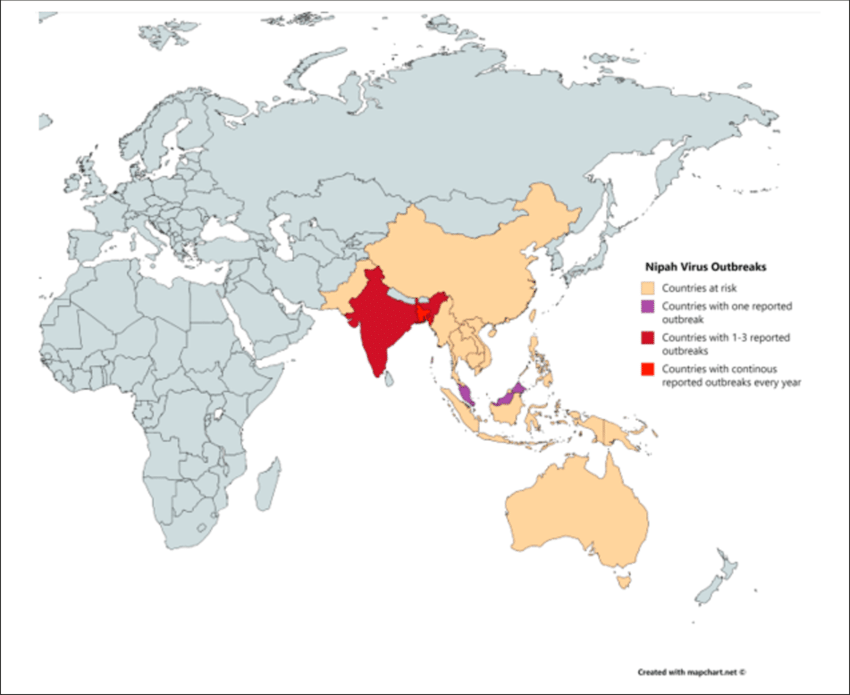
According to media reporting, a student in southern India has died after being infected with the Nipah virus. As of September 14, 2024, 151 people in contact with the student are now being monitored.
India reported its last fatal Nipah case in July 2024.
According to the World Helath Organization (WHO), the first Nipah virus disease outbreak in India was reported in Siliguri in 2001. State authorities alerted Mysuru, Mangaluru, Chamarajanagar, and Kodagu districts in Karnataka, which borders Kerala state.
Currently, the WHO and other health agencies have not issued travel advisories regarding Nipah outbreaks.
The WHO says Nipah has a relatively high case-fatality ratio and is an emerging zoonotic disease of public health importance in the South East Asia and Western Pacific WHO Regions. It was first identified during an outbreak in Malaysia in 1998.
In June 2023, the Coalition for Epidemic Preparedness Innovations invested up to U.S. $100 million in four Nipah vaccine candidate projects. These candidates include live-attenuated and replication-defective recombinant vaccine platforms based on poxviruses, VSV, adenovirus, measles, rabies, and virus-like particles and subunit vaccines.
As of September 17, 2024, neither the U.S. Food and Drug Administration nor the European Medicines Agency has authorized a vaccine candidate for the Nipah virus, but clinical trials are ongoing.
With over 723 deaths from different mpox outbreaks in 14 countries of the African Region, the World Health Organization (WHO) has expanded access to one mpox vaccine.
On September 13, 2024, the WHO announced Bavarian Nordic A/S's MVA-BN (JYNNEOS®) vaccine is the first vaccine against mpox to be added to its prequalification list.
“The WHO prequalification of the MVA-BN vaccine will help accelerate ongoing procurement of the mpox vaccines by governments and international agencies such as Gavi and Unicef to help communities on the frontlines of the ongoing emergency in Africa and beyond,” said Dr. Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, in a press release.
While MVA-BN is currently not licensed for persons under 18, this vaccine may be used “off-label” in infants, children, adolescents, pregnant women, and immunocompromised people. This means vaccine use is recommended in outbreak settings where the benefits of vaccination outweigh the potential risks.
Additionally, the WHO also recommends single-dose use in supply-constrained outbreak situations.
Dr. Rogerio Gaspar, WHO Director for Regulation and Prequalification. “We are progressing with prequalification and emergency use listing procedures with manufacturers of two other mpox vaccines: LC-16 and ACAM2000."
The escalating mpox clade 1 outbreak in the Democratic Republic of the Congo and other countries was declared an emergency by the WHO Director-General on August 14, 2024.
As of mid-Agust 2024, the United States has not reported mpox clade 1 cases, but the JYNNEOS vaccine is commercially available at select clinics and pharmacies.