Search API
Vicebio Ltd. today announced a $100 million Series B financing led by TCGX and others. Vicebio is developing next-generation vaccines for respiratory viruses utilizing its proprietary Molecular Clamp technology, which was discovered at the University of Queensland (UQ).
The Molecular Clamp technology applies to a wide range of viruses, including Respiratory Syncytial Virus (RSV), Human Metapneumovirus (hMPV), Parainfluenza virus, Influenza, and Coronaviruses, as confirmed by promising preclinical and clinical studies.
Vicebio has recently initiated a Phase I clinical trial with VXB-241, its bivalent vaccine targeting RSV and hMPV. Initial clinical readouts of the Phase 1 study are expected to launch in mid-2025.
This financing will also support the acceleration and expansion further development of Vicebio’s multivalent pipeline, including VXB-251, a trivalent vaccine targeting RSV, hMPV, and Parainfluenza Virus 3, a further valency that addresses a significant remaining medical burden in the elderly.
Cariad Chester, Managing Partner at TCGX, said in a September 23, 2024, press release, “Vicebio has a unique capability to advance vaccine products that simultaneously provide robust immune responses against multiple respiratory pathogens. We look forward to working closely with the team to bring these important vaccines to the market.”
Prof. Paul Young, Daniel Watterson, and Keith Chappell at UQ developed the Molecular Clamp proprietary technology.

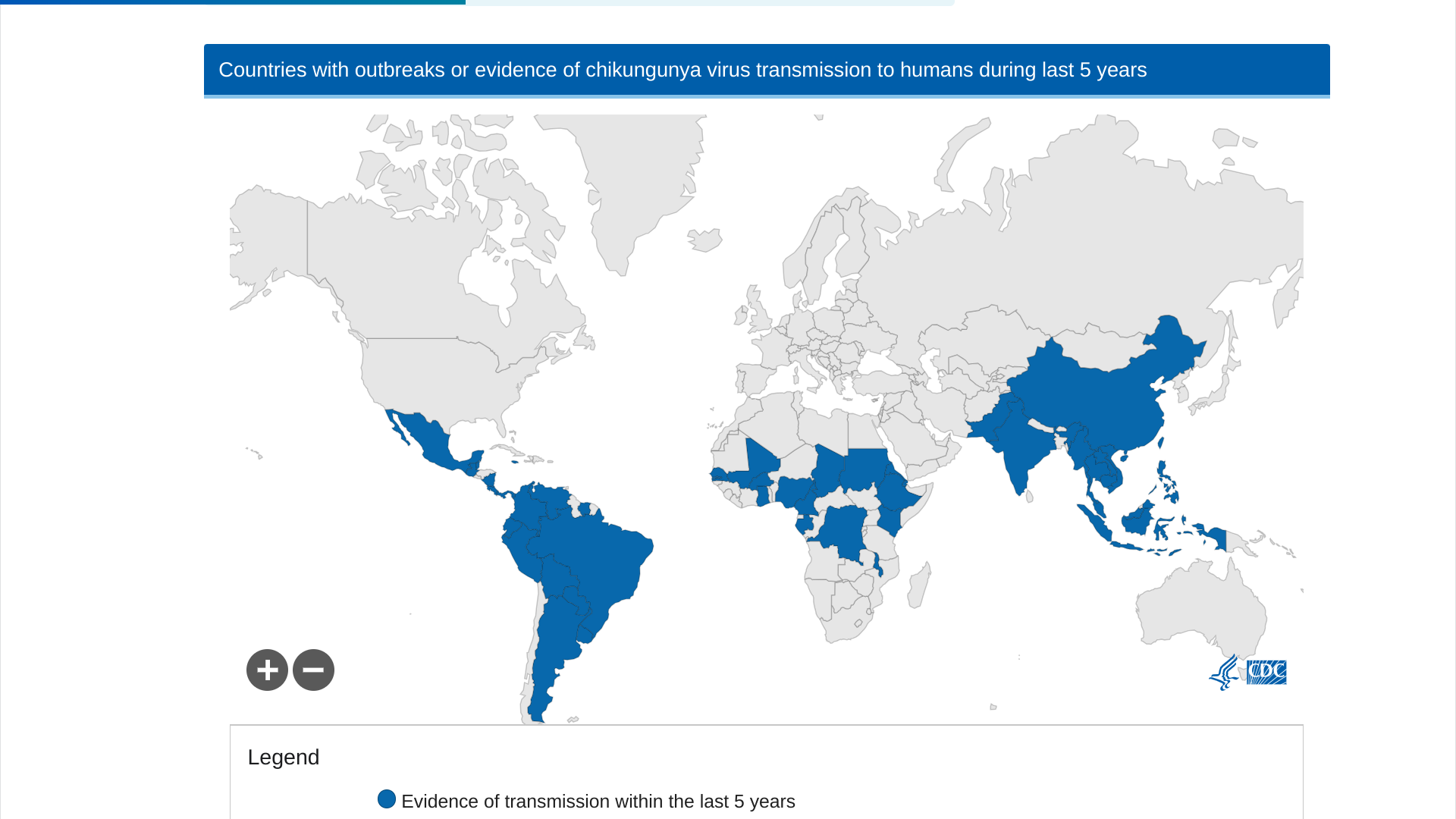
The peer-reviewed journal The Lancet Infectious Diseases recently published interim results of a double-blind, randomized, placebo-controlled phase 3 trial in adolescents of the U.S. FDA-approved, single-dose IXCHIQ® (VLA1553) chikungunya vaccine.
In an article published on September 4, 2024, these researchers concluded that VLA1553 was generally safe and induced seroprotective titers in almost all vaccinated adolescents, with favorable safety data in seropositive adolescents at baseline.
VLA1553 induced seroprotective chikungunya virus neutralizing antibody levels in 247 of 250 (98.8%, 95% CI 96·5–99·8) participants 28 days after vaccination.
This data supports using VLA1553 to prevent diseases caused by the chikungunya virus among adolescents and in endemic areas in the Region of the Americas.
As of September 22, 2024, the Pan American Health Organization reported 390,669 CHIKV cases. Specifically, Brazil has confirmed 170 related deaths this year.
If you are traveling to an area at risk for chikungunya, the U.S. CDC suggests discussing vaccination options with your healthcare provider at least one month before departing abroad.
The Minnesota Department of Health recently affirmed the greater Twin Cities area is experiencing an ongoing outbreak of measles cases, with the virus spreading mainly among unvaccinated children.
The Minneapolis—St. Paul's measles outbreak began in May 2024, and as of September 19, 2024, 51 cases had been confirmed.
Earlier this year, Chicago, Illinois, reported a more significant measles outbreak that impacted 64 people.
Overall, the U.S. CDC has reported 262 measles cases in 32 jurisdictions in 2024.
From a global health-risk perspective, the CDC has issued travel advisories for over 50 countries this year.
Measles is a vaccine-preventable disease. Vaccination is generally recommended for most people, and various vaccines are available at clinics and pharmacies in the U.S.

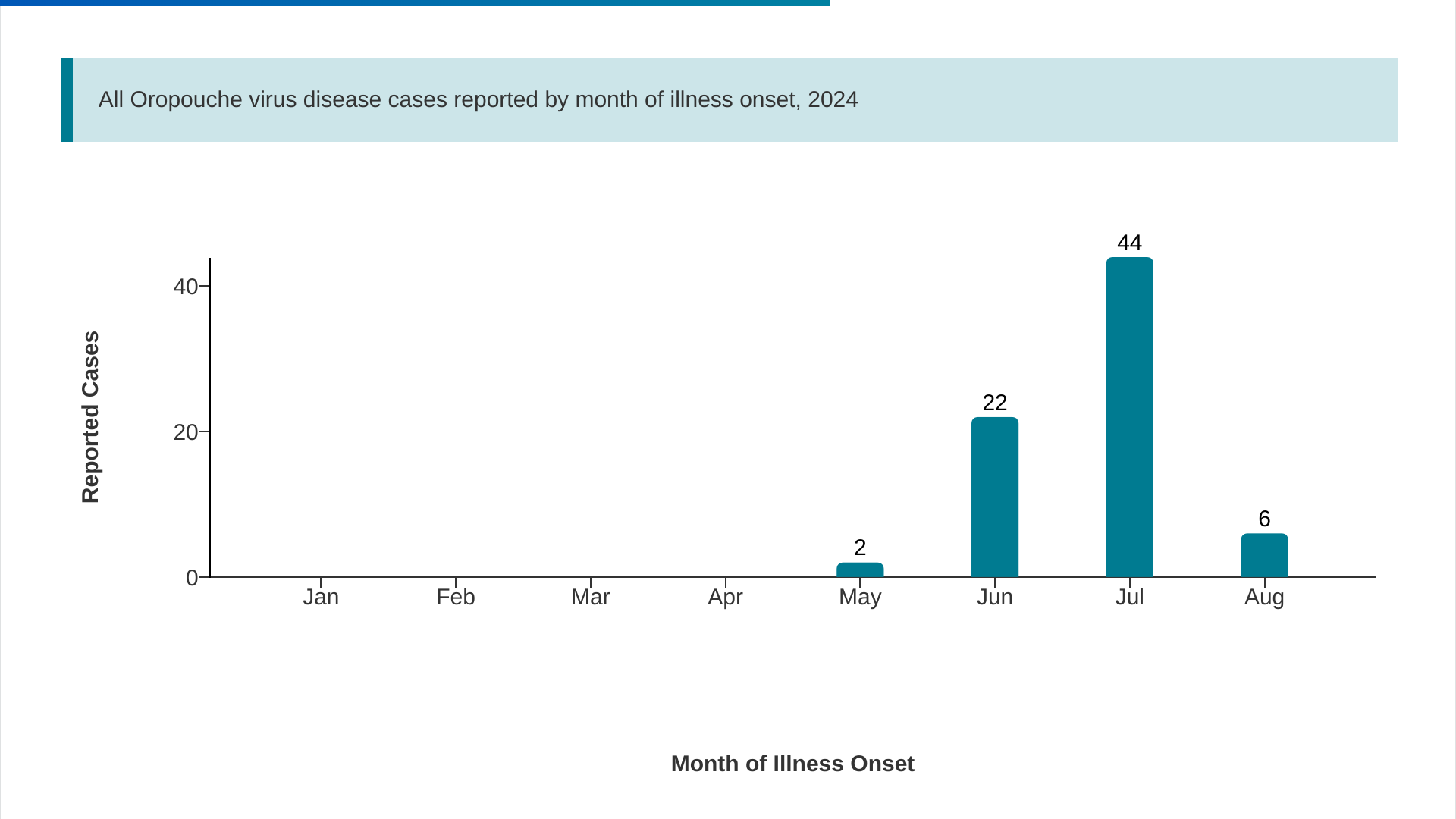
The U.S. Centers for Disease Control and Prevention (CDC) recently reported 22 additional imported cases of the Oropouche virus.
As of September 17, 2024, the total number of cases reported by five U.S. states to 74.
The state of Florida confirmed this week that eleven counties have reported 70 Oropouche cases involving travel to Cuba, which has reported over 500 cases.
The Miami-Dade area (28) has led Florida in reporting cases. The good news is the weekly trends have been decreasing.
In the Region of the Americas, 9,852 cases were confirmed in 2024.
Brazil remains the most affected country, with 7,931 cases and two deaths. Other currently affected countries include Bolivia, Peru, and recently, the Dominican Republic.
The virus is primarily transmitted through the bite of infected midges, small insects that usually bite during the day and inhabit humid areas with organic matter and in forested areas.
Regarding mother-to-child transmission, a total of one fetal death and one case of congenital anomaly have been confirmed in Brazil.
The CDC has confirmed there are not approved vaccines targeting Oropouche virus infections.
GSK plc recently announced topline Phase 3 clinical trial data for a combination regimen of two of its vaccines: the RSV vaccine Arexvy and Shingrix, a market-leading shingles shot.
While GSK did not provide specific clinical trial (NCT05966090) data in its September 18, 2024 announcement, the company did confirm that co-administering Arexvy with Shingrix resulted in a “non-inferior immune response” compared with inoculation with the vaccines at separate visits.
The vaccine combo was also well-tolerated with an “acceptable” safety profile, according to GSK.
This is essential news as both RSV and shingles pose significant health risks to older adults, and these risks only increase with age as the immune system declines.
“With our co-administration studies, GSK is using its science and technology to help remove barriers to adult immunization, by potentially reducing the number of visits to the healthcare offices and pharmacies and ultimately help to get ahead of RSV and shingles,” Led Friedland, GSK’s vice president of scientific affairs and public health, said in the statement.
Results from this trial will be submitted for peer-reviewed scientific publication and used to support regulatory submissions to the U.S. FDA, the European Medicines Agency, and other regulators.

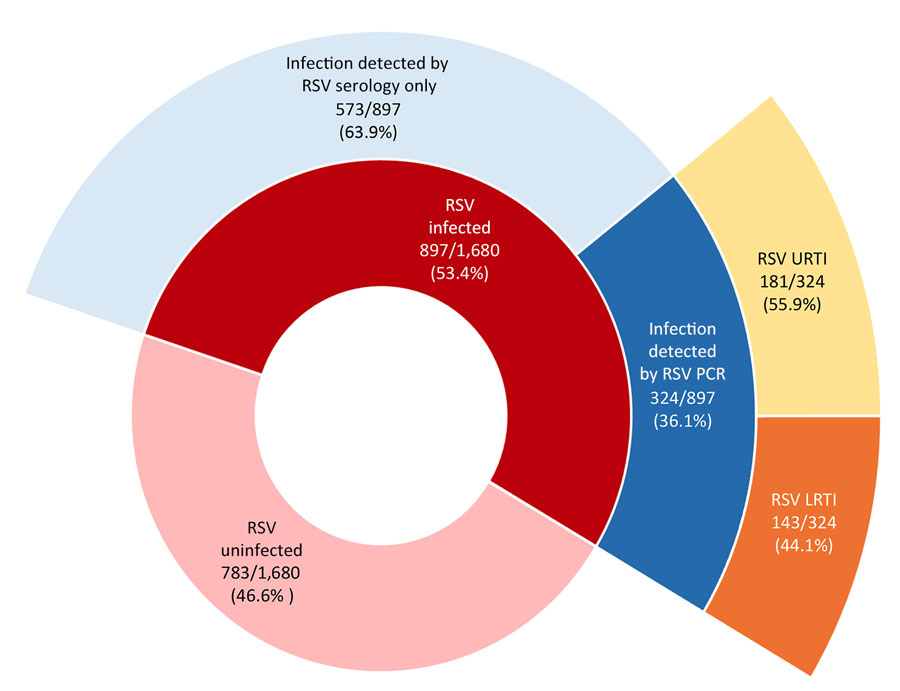
According to a Research Letter, Volume 30, Number 10—October 2024, published in Emerging Infectious Diseases, 53.4% of infants were infected with respiratory syncytial virus (RSV) during infancy, and 2.8% were hospitalized.
The Risk factors for RSV infection during infancy, in order of contribution, were:
- Infant birth month (June vs. referent October, OR 2.42 [95% CI 1.78–3.29]),
- Presence of siblings (OR 1.50 [95% CI 1.22–1.84]),
- Daycare attendance (OR 1.54 [95% CI 1.24–1.93]),
- Increasing percentage below the poverty level in the residential neighborhood (21% vs. 8%; OR 1.19 [95% CI 1.05–1.36]), and
- Public insurance (OR 1.28, 95% CI 1.02–1.62).
The researchers determined secondhand smoke exposure, sex, ever being breastfed, maternal asthma, and study year were not significantly associated with the likelihood of infant RSV infection.
In conclusion, 'our data are important estimates of RSV disease's burden and infection risk factors in healthy-term infants. Our findings provide a benchmark to monitor the effects of recently available maternal vaccines in the United States and extended half-life monoclonal antibodies (Beyfortus™) for preventing severe RSV illness in early life.'
As of 2023, the U.S. CDC says that infants and children who are recommended to receive Beyfortus should be immunized as quickly as possible.