Search API
Hong Kong's Centre for Health Protection (CHP) announced today it is closely monitoring five human cases of avian influenza A(H5N6) in the China Mainland. Two men from different cities in Sichuan province died from their infections.
An earlier case in 2022 involved a 43-year-old woman living in Huizhou in Guangdong Province.
Since 2014, China has reported 63 human cases, but the country reported a sharp rise in cases (36) last year.
The virus has been detected in birds in four Asian countries, but China and Laos are the only ones that have reported human cases.
Whenever avian influenza viruses are circulating, there is a risk for sporadic infection and small clusters of human cases due to exposure to infected poultry or contaminated environments, says the U.S. CDC.
H5N6 infections are often severe or fatal.
Travelers to the Mainland or other affected areas must avoid visiting wet markets, live poultry markets, or farms.
They should also avoid purchasing live or freshly slaughtered poultry and avoid touching poultry/birds or their droppings. In addition, they should strictly observe personal and hand hygiene when visiting any place with live poultry.
And travelers returning from affected areas should consult a doctor promptly if symptoms develop and inform the doctor of their travel history for prompt diagnosis and treatment of potential diseases.
The annual flu shot does not protect people from avian influenza viruses, says the CDC.

California-based Vir Biotechnology, Inc. today announced an expansion of its partnership with the Bill & Melinda Gates Foundation to include the advancement of innovative platform technologies in the development of broadly neutralizing antibodies designed to provide a "vaccinal effect" for the treatment of HIV and prevention of malaria.
"Vir's partnership with the Bill & Melinda Gates Foundation has been a formative and essential part of our company history beginning with our T-cell vaccine program targeting HIV and tuberculosis," said George Scangos, Ph.D., CEO of Vir Biotechnology, in a press release issued on January 13, 2022.
"This expanded collaboration into a second platform technology supports our shared goal of developing innovative solutions for prevention and treatment of global infectious diseases."
"We look forward to applying the lessons learned thus far in COVID-19, chronic hepatitis B virus infection and influenza to advance our efforts toward curing HIV and preventing malaria."
This novel program combines Vir's extensive immunologic and virologic expertise with the Gates Foundation's long-standing global health leadership to address two of the world's most challenging infectious diseases.
"Even though HIV has gone from being a near-term fatal disease to a chronic condition for those who have access to effective antiviral therapies, there remains a significant unmet need for new advances that could enable durable antiretroviral-free suppression of HIV," commented Mike McCune, M.D., Ph.D., head of the HIV Frontiers Program at the Gates Foundation.
"The foundation is pleased to support the development of this novel vaccinal antibody technology that has the potential to result in such suppression and is committed to advancing access to this cutting-edge innovation globally."
The Gates Foundation has committed a $40 million equity investment and a $10 million grant to support this effort.
Vir Biotechnology is a commercial-stage immunology company located in San Francisco, CA, focused on combining immunologic insights with cutting-edge technologies to treat and prevent serious infectious diseases.

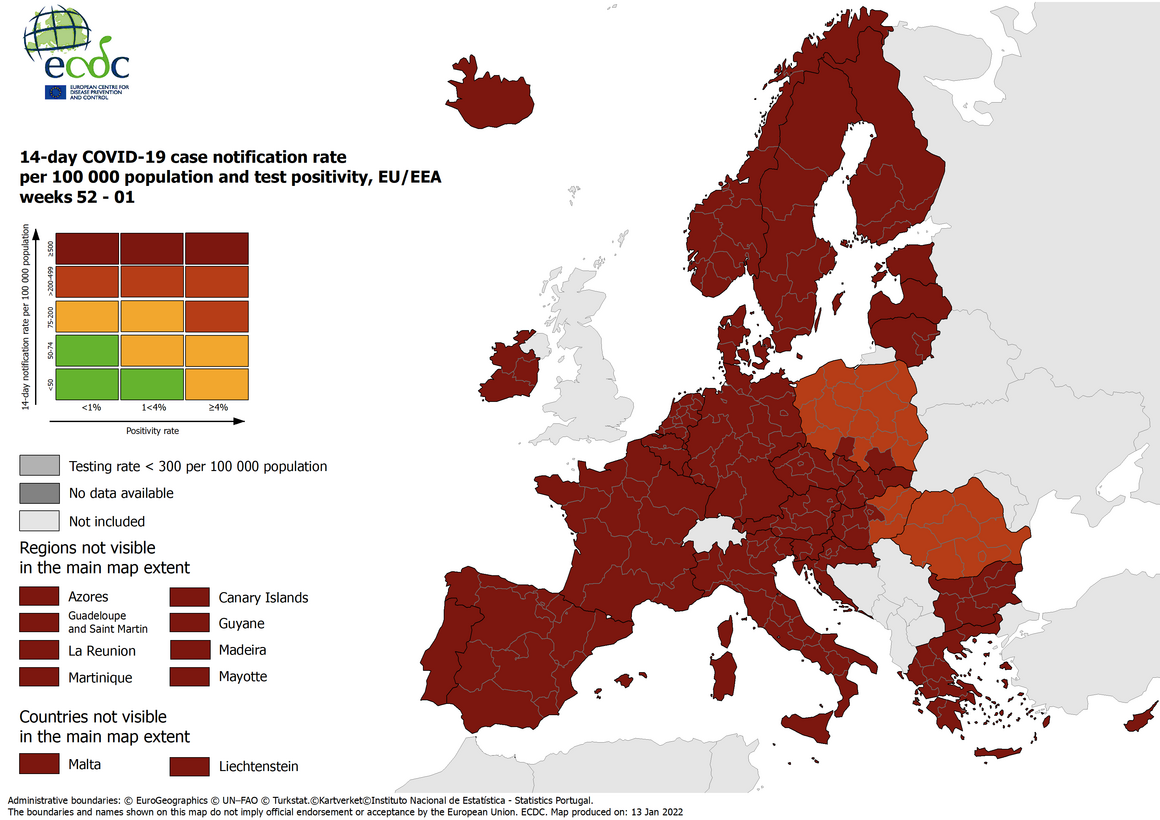
The European Centre for Disease Prevention and Control (ECDC) published updated, color-code maps supporting the Council Recommendation on a coordinated approach to travel measures in the EU.
Areas are marked in the following colors:
- Red: if the 14-day cumulative COVID-19 case notification rate ranges from 75 to 200 and the test positivity rate of tests for COVID-19 infection is 4% or more, or if the 14-day cumulative COVID-19 case notification rate is more than 200 but less than 500.
- Dark red: if the 14-day cumulative COVID-19 case notification rate is 500 or more
- Grey: if there is insufficient information or if the testing rate is lower than 300 cases per 100 000.
The ECDC also published a data dashboard identifying SARS-CoV-2 variants of interest and concern in Europe.
As of January 13, 2022, the ECDC reported 69% of Europeans had been fully vaccinated against cOVID-19.
California-based Gritstone bio, Inc. announced that the first patient had been enrolled in the Phase 2/3 GRANITE-CRC-1L trial.
This clinical trial evaluates the individualized neoantigen vaccine GRANITE combined with immune checkpoint blockade for the first line (1L) maintenance treatment of newly diagnosed patients with metastatic, microsatellite-stable colorectal cancer (MSS-CRC).
GRANITE is an individualized neoantigen-based immunotherapy and uses a priming adenoviral vector (GRT-C901) and self-amplifying mRNA (samRNA) vector (GRT-R902) to deliver individualized immunotherapy containing the relevant neoantigens. GRANITE was granted Fast Track designation by the U.S. FDA for treating MSS-CRC.
Gritstone’s neoantigen-based immunotherapies are engineered to elicit a significant T-cell response (particularly CD8+ cytotoxic T cells) against mutation-derived tumor-specific neoantigens identified by the company using its proprietary Gritstone EDGE™ artificial intelligence platform.
“Building on the success of our GRANITE program, which continues to demonstrate extended survival in multiple end-stage colorectal cancer patients, we are excited to launch this randomized, open-label Phase 2/3 trial to evaluate earlier use of GRANITE as a maintenance treatment in newly diagnosed patients with metastatic, microsatellite-stable colorectal cancer,” said Andrew Allen, M.D., Ph.D., Co-founder, President and CEO of Gritstone, in a press release issued on January 13, 2022.
“We expect to report initial Phase 2 data from the GRANITE-CRC-1L trial in mid-2023.”
Gritstone bio, Inc. is a clinical-stage biotechnology company located in Emeryville, CA, developing the next generation of immunotherapies against multiple cancer types and infectious diseases.

Three studies presented during the 63rd American Society of Hematology Annual Meeting and Exposition on January 11, 2022, spotlights novel approaches to screening for and treating blood diseases.
As well as an unexpected potential association between a blood abnormality and Alzheimer's disease.
The first study demonstrates how the use of high-sensitivity screening techniques for the early detection of blood abnormalities may identify people at high risk for multiple myeloma earlier – especially Black patients and those with a first-degree relative with the disease – giving them more timely access to treatment.
The second reveals a surprising possible association between a fairly common blood abnormality in older adults and a reduced risk for Alzheimer's disease, the most common cause of dementia.
And in the third – the most extensive study to date of gene therapy for a blood disorder – investigators report on a novel treatment strategy with the potential to dramatically improve the quality and quantity of life for patients with a severe form of an inherited blood disease.
"Each of these studies is compelling in its own way," said press briefing moderator Joseph Mikhael, M.D., of the Translational Genomics Research Institute.
"The first presents a strong case for screening people at high risk for multiple myeloma."
"This could have important health equity implications because multiple myeloma is twice as common among African Americans as in the general population."
To ensure adequate representation of people of African descent in the study population, Dr. Ghobrial and colleagues also identified and screened Black people who had contributed blood samples to a sizeable biological specimen repository, the Mass General Brigham Biobank in Boston.
The investigators report interim screening findings for 7,622 participants in the current study, including 2,439 Black people.
"Ours is the largest cohort of Black people to be recruited for a myeloma screening study and the first prospective study to actively recruit people at high risk for multiple myeloma, follow them over time to estimate the prevalence of MGUS accurately, and explore outcomes for patients with this precursor condition," said Dr. Ghobrial.

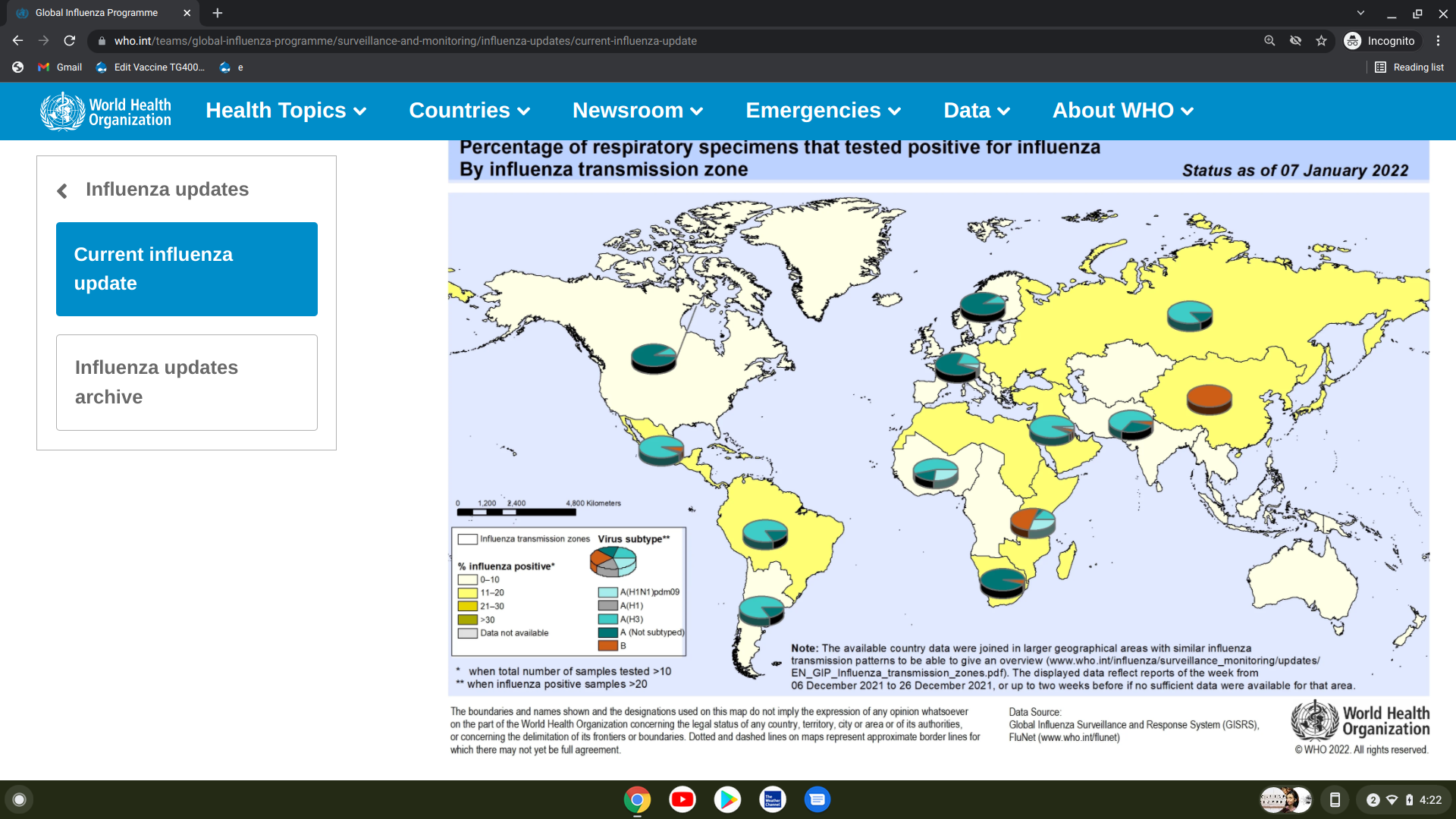
The World Health Organization (WHO) issued Influenza Update N° 410 on January 10, 2022, which says 'influenza activity remains low but continued to increase in the temperate zones of the Northern Hemisphere, such as the U.S.'
In the temperate zones of the Southern Hemisphere, influenza activity remained low overall, although increased detections of influenza A(H3N2) were reported in temperate South America.
The WHO National Influenza Centres and other laboratories from 110 countries, areas, or territories reported data to FluNet as of 2022-01-07 11:00:29 UTC.
These laboratories tested more than 522,595 specimens during the last period.
A total of 27,153 were positive for influenza viruses, reflecting a 0.05% positivity rate.
Of the confirmed specimens, about 73% were typed as influenza A. And of the sub-typed influenza A viruses, 95.6% were influenza A(H3N2).
The WHO advised 'Clinicians to consider influenza in differential diagnosis especially for high-risk groups for influenza, and test and treat according to national guidance.'
From a regional perspective, the WHO disclosed:
- In North America, influenza virus detections of predominately A(H3N2) among the subtyped increased, and hospitalizations are increasing but remain low overall.
- RSV activity decreased in the USA and Canada.
- In Europe, Influenza A(H3N2) was predominated.
- In East Asia, influenza activity continued increasing in China, while influenza illness indicators and activity remained low in the rest of the subregion.
- In the Caribbean and Central American countries, some countries increased influenza A(H3N2) and B virus detections.
- In tropical South America, influenza A(H3N2) detections increased overall, with severe acute respiratory infection levels were reported at extraordinary levels in Bolivia (Plurinational State).
- In tropical Africa, overall influenza activity continued on a decreasing trend.
- In Southern Asia, influenza virus detections of predominately influenza A(H3N2) increased overall, although decreasing in a few countries.
- In South-East Asia, sporadic influenza detections were reported in the Philippines.
Within the U.S., the Centers for Disease Control and Prevention (CDC) released its weekly update on January 7, 2022, saying 'influenza activity is increasing, with the eastern and central parts of the country seeing the majority of viruses reported.'
The majority of influenza viruses detected are A(H3N2).
So far, most of the H3N2 viruses are genetically closely related to the flu vaccine virus, but some antigenic differences have developed as H3N2 viruses have continued to evolve.
Separately on January 12, 2022, the CDC's Advisory Committee on Immunization Practices reviewed updated presentations, such as 'Influenza Vaccines for Older Adults,' presented by Lisa Grohskopf, CDC Vaccine Policy Unit.
Additional flu vaccine news is published on this Precision Vaccinations webpage.
Bloomberg Law confirmed on January 11, 2022, European Medicine Agency (EMA) regulators warned that frequent COVID-19 vaccinations could adversely affect the human immune response.
Boosters "can be done once, or maybe twice, but it's not something that we can think should be repeated constantly," Marco Cavaleri, the EMA head of biological health threats and vaccines strategy, said at a recent press briefing.
"We need to think about how we can transition from the current pandemic setting to a more endemic setting."
This EMA advice follows different tactics/advice announced by Israel, the United Kingdom (U.K.), and the World Health Organization, which stated on January 11, 2022, 'a vaccination strategy based on repeated booster doses of the original vaccine composition is unlikely to be appropriate or sustainable.'
In December 2021, Israel became the first country to administer a second booster, or fourth mRNA vaccination, to Israelis over the age of 60.
However, the U.K. stated there is no need for a second booster.
The Joint Committee on Vaccination and Immunisation chair of COVID-19 immunization, Professor Wei Shen Lim, said in a media statement issued on January 7, 2022: 'The current data shows the booster dose continues to provide high levels of protection against severe disease, even for the most vulnerable older age groups.'
'For this reason, the committee has concluded there is no immediate need to introduce a second booster dose.'
In the U.S., the Food and Drug Administration recently amended the emergency use authorization for mRNA COVID-19 vaccines to shorten the time between completing a two-dose primary series of the vaccine and a booster dose to at least five months for individuals 18 years of age and older.
Peter Marks, M.D., Ph.D., director of the FDA's Center for Biologics Evaluation and Research, commented in a media statement, "We encourage everyone to get vaccinated—it's never too late to get your COVID-19 vaccine or booster."
The EMA currently authorizes five COVID-19 vaccines.
Maryland-based Novavax Inc.'s Nuvaxovid vaccine was recently authorized for adults in Europe.
The EMA is a decentralized agency of the European Union located in Amsterdam. It began operating in 1995 and is responsible for the scientific evaluation, supervision, and safety monitoring of medicines in Europe.

California-based Polynoma LLC today announced that it had reached an agreement with the U.S. Food and Drug Administration (FDA) under a Special Protocol Assessment (SPA) on a pivotal Phase 3 clinical study of seviprotimut-L, Polynoma's melanoma cancer vaccine.
This SPA is for the adjuvant treatment of patients 60 years and younger with Stage IIB or IIC melanoma following definitive surgical resection to improve recurrence-free survival.
Seviprotimut-L previously received Fast Track designation from the U.S. FDA.
The final analysis of Part B1 data from the Melanoma Antigen Vaccine Immunotherapy Study (MAVIS) was recently published in the Journal for ImmunoTherapy of Cancer.
A subgroup analysis of patients receiving seviprotimut-L with AJCC Stage IIB/IIC melanoma, under age 60 with a median follow-up time of 45.8 months (3.8 years), showed clinically significant improvement in recurrence-free survival (RFS), reducing the risk of disease recurrence or death by 68% (HR=0.32; 95% CI, 0.121, 0.864) compared to patients receiving placebo.
Additionally, RFS was more favorable in patients under age 60 with ulcerated melanomas (HR 0.21; 95% CI: 0.065-0.702), and there was a trend toward improved overall survival (HR 0.34; 95% CI: 0.117, 0.975) for patients that received seviprotimut-L compared to those receiving placebo.
There were no immune-mediated AEs, or other treatment-related serious AEs observed.
"Vaccination with seviprotimut- L has an advantage of having very low toxicity, without significant immune-related adverse events and no significant increase in toxicity over placebo," said Craig L. Slingluff, Jr., M.D., Professor of Surgery and Director of the Human Immune Therapy Center and lead author of the JITC research paper on MAVIS, in a press statement issued on January 11, 2022.
"If the definitive evaluation of this vaccine therapy confirms clinical benefit in patients with Stage IIB/IIC melanoma, particularly those aged 60 and younger, the low toxicity of this approach will be a valuable option for these patients."
Seviprotimut-L is an allogeneic, polyvalent, partially purified shed melanoma antigen vaccine derived from three proprietary human melanoma cell lines. Seviprotimut-L stimulates humoral and cellular immune responses.
Melanoma-associated antigens (MAAs) found in seviprotimut-L are taken up by antigen-presenting cells (dendritic cells), which then activate the production of antigen-specific cytotoxic T-lymphocytes (CTLs) as well as develop antibody responses against MAAs.
These CTLs and antibodies then recognize and act on tumor cells expressing the MAAs on their surfaces, causing cell death.
Seviprotimut-L is currently developing for the adjuvant treatment of patients with Stages IIB and IIC melanoma following definitive resection.
Polynoma LLC is a U.S. immuno-oncology focused biopharmaceutical company headquartered in San Diego, CA, and is a wholly-owned subsidiary of CK Life Sciences Int'l., Inc.

The U.S. Centers for Disease Control and Prevention (CDC) issued a Level 4 Advisory: Very High Level of COVID-19 in Canada on January 10, 2022. The CDC says 'avoid traveling to Canada.'
And if you must travel to Canada, 'make sure you are fully vaccinated before travel.' However, because of the current situation in Canada, even fully vaccinated travelers may be at risk for getting and spreading COVID-19 variants.'
If you decide to travel to Canada, the U.S. Department of State suggests visiting the U.S. Embassy's webpage regarding COVID-19. Fully vaccinated foreign travelers can travel to the U.S. across the Northern border with Canada.
Separately, the Canadian government issued detailed data about the spread of the virus on January 11, 2022. Of the 13 Canadian jurisdictions reporting updates, no new deaths were reported in six provinces and territories in the past 24 hours.
The U.S. suggests enrolling in the Smart Traveler Enrollment Program to receive Alerts and make it easier to locate American citizens in an emergency.
Land border restrictions for travel to the U.S. remain in effect through January 21, 2022, and maybe extended.
If you are overseas and your U.S. passport expired on or after January 1, 2020, you may be able to use your expired U.S. passport to return directly to the United States until March 31, 2022. See this State Department webpage to determine if you qualify for this exception.
The U.S. Department of State updated its passport FAQs on January 10, 2022.

