Search API
The Annals of Internal Medicine published the results from a peer-reviewed systematic review on January 18, 2022, that identified 19 studies addressing the use of follow-up colonoscopy to assess the presence of colonic neoplasia after an episode of presumed acute left-sided colonic diverticulitis.
Clinicians and patients face several decisions in planning appropriate evaluation and management after acute left-sided colonic diverticulitis.
The American College of Physicians (ACP) developed this guideline to provide clinical recommendations on the role of colonoscopy for diagnostic evaluation of colorectal cancer (CRC) after a presumed diagnosis of acute left-sided colonic diverticulitis and on the role of pharmacologic, nonpharmacologic, and elective surgical interventions to prevent recurrence after initial treatment of acute complicated and uncomplicated left-sided colonic diverticulitis.
Important outcomes, including colonoscopy complications or failed or incomplete colonoscopy, were rarely reported in primary studies.
High-certainty evidence showed that very few had complications among 1,253 patients in five observational studies, such as bleeding or perforation (0.2% [CI, 0.04% to 0.64%]).
High-certainty evidence from observational studies showed that 3.7% (CI, 2.7% to 4.9%) of patients with acute left-sided colonic diverticulitis undergoing colonoscopy six weeks to 1 year after hospital discharge had a failed or incomplete colonoscopy.
The target audience for this ACP guideline is all clinicians, and the target patient population is adults with recent episodes of acute left-sided colonic diverticulitis.
China-based CStone Pharmaceuticals today announced on January 19, 2022, that a phase 3 clinical study of sugemalimab for the first-line treatment of metastatic (stage IV) non-small cell lung cancer (NSCLC) met the overall survival (OS) endpoint.
This study's results demonstrated that sugemalimab combined with chemotherapy showed statistically significant and clinically meaningful OS improvement in patients.
Based on the previously reported impressive progression-free survival (PFS) data, the National Medical Products Administration of China approved sugemalimab in combination with chemotherapy for the first-line treatment of patients with metastatic squamous and non-squamous NSCLC in December 2021.
In addition, sugemalimab is being investigated in several ongoing clinical trials, including one Phase 2 registrational study for lymphoma and four Phase 3 registrational studies in stage IV NSCLC, stage III NSCLC, gastric cancer, and esophageal cancer, respectively.
Professor Caicun Zhou, Principal Investigator of the GEMSTONE-302 registrational clinical study of sugemalimab and Director of the Department of Oncology, Shanghai Pulmonary Hospital, Tongji University, said in a press release, "Globally, the mortality of lung cancer ranks first among all malignant tumors. The goal of first-line treatment for advanced lung cancer is to maximally prolong survival benefits for patients and to delay disease progression."
"The prespecified OS analysis data further confirmed that sugemalimab combined with chemotherapy provided durable survival benefits to patients."
"Sugemalimab has the potential to reshape the first-line treatment landscape of advanced NSCLC and could become the preferred immune-oncology therapy for the treatment of advanced NSCLC."
The anti-PD-L1 monoclonal antibody (mAbs) sugemalimab was discovered by CStone using OmniRat® transgenic animal platform, which allows the creation of fully human antibodies in one step.
As a result, Sugemalimab is a fully human, full-length anti-PD-L1 immunoglobulin G4 mAbs, allowing a reduced risk of immunogenicity and toxicity for patients, a unique advantage over similar drugs.
CStone recently formed a strategic collaboration agreement with New York-based Pfizer Inc., including the development and commercialization of sugemalimab in mainland China and a framework to bring additional Oncology medicines to the greater China market.
Established in 2015, CStone Pharmaceuticals (HKEX: 2616) is a biopharmaceutical company focused on researching, developing, and commercializing innovative immuno-oncology and precision medicines to address the unmet medical needs of cancer patients in China and worldwide.
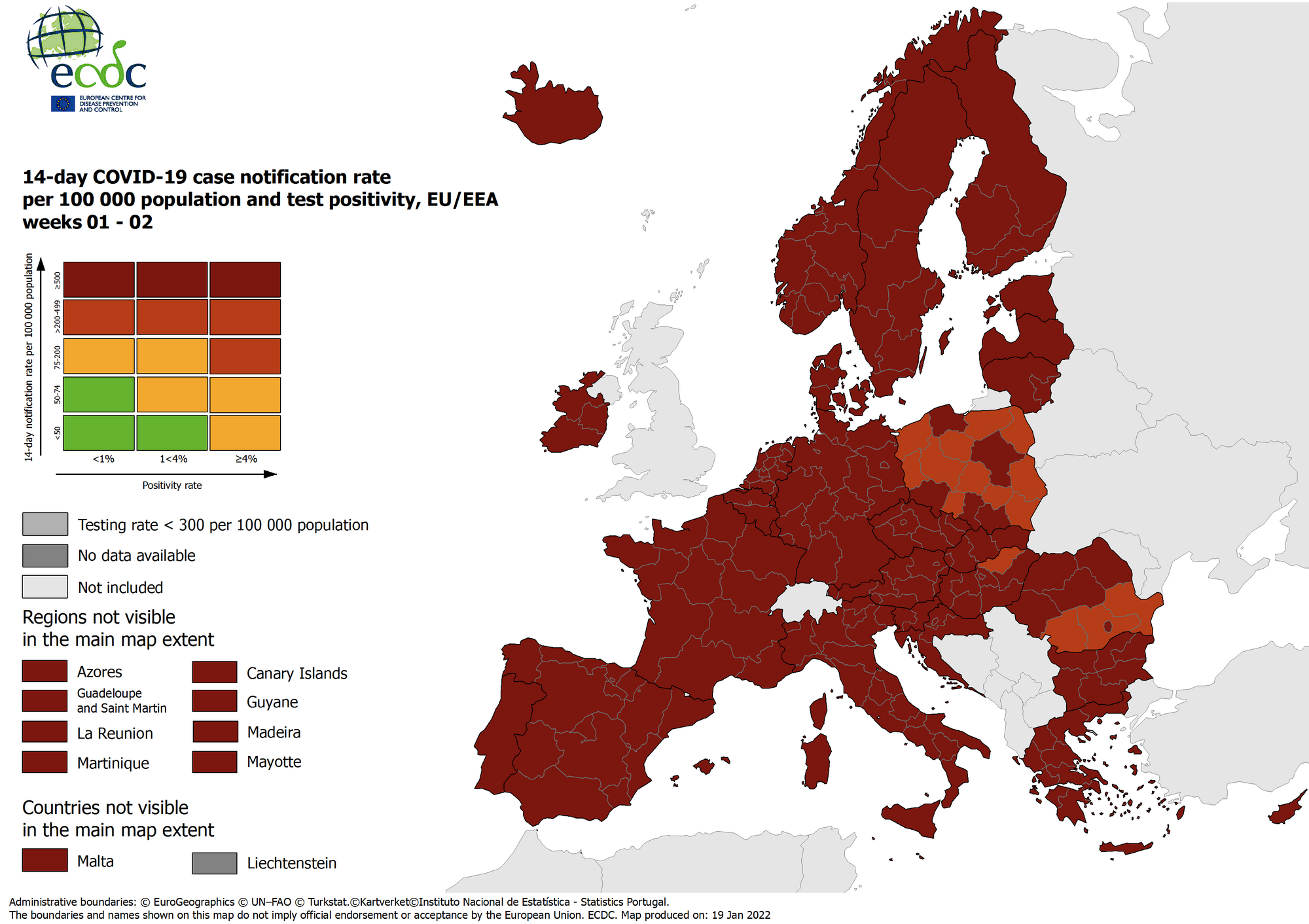
The European Centre for Disease Prevention and Control (ECDC) published today updated color-coded maps that highlight the ongoing COVID-19 pandemic's impact in Europe.
Data for the maps in support of the Council Recommendation on a coordinated approach to the restriction of free movement in the EU/EEA
As of January 20, 2022, the ECDC maps are based on data reported by the EU Member States every Tuesday.
The Red and Dark Red colored countries are reporting significant increases in SARS-CoV-2 coronavirus cases, most related to the Omicron variant.
From a COVID-19 vaccination perspective, the ECDC recently reported over 69% of people in twenty-nine Europeans had been fully vaccinated.
This compares with about 63% of the U.S. population being fully vaccinated with an Approved or Authorized COVID-19 vaccine.
Hong Kong-based HUTCHMED today announced it had initiated a Phase I trial in China of HMPL-653, an investigational novel, highly selective, and potent colony-stimulating factor 1 receptor (“CSF-1R”) inhibitor.
The first patient received their first dose on January 18, 2022.
Currently, no CSF-1R inhibitor has been approved in China. However, there is a high unmet need for an effective and safe treatment for these patients.
The Phase I trial is a multicenter, open-label, single-arm study to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of HMPL-653 in treating patients with advanced or metastatic solid tumors and tenosynovial giant cell tumors (“TGCT”).
HMPL-653 is an investigational novel, highly selective, and potent CSF-1R inhibitor designed to target malignant driven tumors as a monotherapy or in combination with other drugs.
CSF-1R is usually expressed on the surface of macrophages and can promote growth and differentiation of macrophages after binding with its ligand, CSF-1.
Several previous studies have shown that blocking the CSF-1R signaling pathway could effectively modulate the tumor microenvironment, relieve tumor immunosuppression, and synergize with other anti-cancer therapies such as immune checkpoint inhibitors to achieve tumor inhibition.
TGCT is a rare soft tissue tumor caused by abnormal proliferation and inflammation of giant cells, monocytes, and inflammatory cells.
The incidence of TGCT is approximately between 1.8 and 50 per 1 million people.
The expression of CSF-1 mainly characterizes these tumors.
Surgery is the standard treatment for TGCT patients. However, among patients with diffuse or recurrent/refractory TGCT, tumors are wrapped in peripheral organs such as bone, tendon, ligament, and joint, making removal by surgery difficult.
The recurrence rate of diffuse-type cases is estimated to be 21% to 50%.
HUTCHMED currently retains all rights to HMPL-653 worldwide.
HUTCHMED (Nasdaq/AIM: HCM; HKEX:13) is an innovative, commercial-stage biopharmaceutical company with operations in Florham Park, New Jersey.
As of February 9, 2022, the latest anti-SARS-CoV-2 monoclonal antibody (mAbs) breaking news is published at Precision Vaccinations.
There are anti-SARS mAbs products Authorized by the U.S. FDA to treat certain patients. Furthermore, the U.S. NIH continues to identify which mAbs is most effective against the Omicron variant.
Since September 13, 2021, the U.S. government has distributed about 3.3 million mAbs treatments to states, territories, and agencies.

The JAMA Network published the finding from an Orginal Investigation on January 18, 2022. For children responding to initial treatment for outpatient community-acquired pneumonia (CAP), a 5-day antibiotic strategy was superior to a 10-day plan.
Led by investigators with Vanderbilt University Medical Center, the Short-Course Outpatient Therapy of CAP (SCOUT-CAP) randomized phase 4 clinical trial of 380 children, a 5-day treatment strategy resulted in similar outcomes with fewer antibiotic days.
The 5-day strategy was associated with a 69% probability of a more desirable outcome and a significantly lower abundance of antibiotic resistance genes for the primary composite outcome.
This shortened approach resulted in similar clinical responses and antibiotic-associated adverse effects while reducing antibiotic exposure and resistance.
The study authors say that while these findings are consistent with previous studies, their study makes a case for superiority by examining antibiotic-associated adverse effects and the associations between treatment duration and its impact on the reservoir of resistance genes in the respiratory tract.
"SCOUT-CAP is the first clinical trial to use this particular innovative trial design that takes into account both the response to therapy and the potential side effects of that therapy," the corresponding author Buddy Creech, M.D., director of the Vanderbilt Vaccine Research Program, said in a press release.
"Using this approach in future trials may allow us to optimize therapy for a number of infectious diseases in children and adults," he said.
This project was funded, in part, with funds from the National Institute of Allergy and Infectious Diseases Vaccine Treatment and Evaluation Unit at Vanderbilt University Medical Center. The research team disclosed no conflicts of interest.

California-based ImmunityBio, Inc. announced interim results in its metastatic pancreatic cancer trial (QUILT 88), showing that the overall survival rate for patients doubled compared to the historical survival rate of three months after two prior lines of therapy.
The data of the Phase 2 trial studying combination immunotherapy (Nant Cancer Vaccine) also show treatment-related serious adverse events were uncommon (8%), and no treatment-related deaths were reported.
Based on these findings, ImmunityBio plans to meet with the U.S. FDA in 2022 to discuss the path for the Approval of combination therapies for pancreatic cancer.
"There are thousands of patients in advanced stages of this disease, and there are few, if any, treatment options for them," commented Patrick Soon-Shiong, M.D., Founder, and Global Chief Scientific and Medical Officer of ImmunityBio, Inc, in a press statement issued on January 18, 2022.
"Based on this encouraging data from our QUILT 88 trial, we are hopeful that our Nant Cancer Vaccine can potentially address this unmet need."
"What's more, we designed this therapeutic candidate to be administered in an outpatient setting, making it more accessible to future patients than traditional immune checkpoint inhibitors."
The results were presented today at the American Society of Clinical Oncology Gastrointestinal conference being held virtually.
To date, 27% of third-line or greater patients (17/63) remain on study. The median overall survival in this highly advanced group of patients, who failed two to six prior lines of treatment, is 5.8 months (95% CI: 3.9, 6.9 months), exceeding the approximately three-month historical median overall survival.
Of the 63 patients, 30 (48%) had progressed after two prior lines of therapy.
The median overall survival in this group was 6.3 months (95% CI: 5.0, 9.8 months), more than doubling the overall historical survival.
The company has expanded enrollment in the third-line or greater cohort on the strength of this early data and significant unmet medical need.
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States and has one of the highest mortality rates of all major cancers, taking nearly 50,000 lives in the U.S. every year.
Located in Culver City, CA, ImmunityBio's clinical pipeline consists of 21 clinical trials—13 of which are in Phase II or III development—across 12 indications in solid and liquid cancers and infectious diseases.

Due to operational concerns associated with the planned deployment of 5G mobile network services near certain airports, the international airline Emirates announced on January 18, 2022, it will be suspending flights to specific U.S. destinations until further notice.
The impacted airports include Boston (BOS), Chicago (ORD), Dallas Fort Worth (DFW), Houston (IAH), Miami (MIA), Newark (EWR), Orlando (MCO), San Francisco (SFO), and Seattle (SEA).
However, Emirates flights to New York JFK, Los Angeles (LAX), and Washington DC (IAD) continue to operate as scheduled.
Emirates stated 'Affected customers do not need to call immediately for rebooking. Instead, customers can hold on to their Emirates ticket and when flights resume, get in touch with their travel agent or booking office to make new travel plans.'
'We are working closely with aircraft manufacturers and the relevant authorities to alleviate operational concerns, and we hope to resume our U.S. services as soon as possible.'
According to media reporting, Air India, All Nippon Airways, and Japan Airlines have also suspended most routes to the United States as of January 19.
Airlines have previously stated that the 'deployment of 5G could interfere with aircraft navigation systems and impact operations.'

The European Travel Information and Authorisation System (ETIAS) will soon change the way millions of people travel to Europe. According to Frontex's recent data, about 20% of travelers will need to apply for ETIAS Visa Waiver.
Travel to Europe began to change when the legal procedures to pass the ETIAS were introduced in 2016, and the system is expected to be fully operational in 2024.
ETIAS will have a significant impact on international mobility.
Currently, EU border officials carry out checks on visitors when they are already at the border.
They assess whether people traveling to Europe without a visa could threaten security or public health.
ETIAS will allow these checks to be made before travelers depart, improving EU security and making the border crossing a smoother process. As a result, the EU expects around 97% of applications will be digitally approved within 96 hours.
Thirty European countries are in the process of establishing their ETIAS National Units.
In the news release, Frontex Executive Director Fabrice Leggeri explained, "ETIAS is an important part of the digitalization process of our border management, helping to ensure the resumption of international mobility in the post-COVID world with higher security and safety standards."
According to preliminary figures announced by Frontex on January 11, 2022, the total number of illegal border crossings in 2021 was just short of 200,000, the highest number since 2017.
This is an increase of 36% compared with 2019 and a rise of 57% compared with 2020.
This trend suggests that factors other than the lifting of restrictions on global mobility are the cause of increased migratory pressure.
Frontex, the European Border, and Coast Guard Agency, is an essential part of Europe's efforts to safeguard the area of freedom, security, and justice. Frontex's support at the external borders helps guarantee free movement without internal borders checks that many of us take for granted.