Search API
New Jersey-based Merck and Ridgeback Biotherapeutics today announced that data evaluating LAGEVRIO™(molnupiravir), an oral antiviral COVID-19 medicine, will be presented at the 2022 European Congress of Clinical Microbiology & Infectious Diseases.
The presentation (Abstract #4514) includes final analyses evaluating virologic outcomes throughout and following a five-day course of LAGEVRIO as part of the Phase 3 MOVe-OUT clinical trial.
In participants with infectious virus isolated at baseline and for whom post-baseline infectivity data were available, molnupiravir was associated with more rapid elimination of infectious virus than placebo.
- At Day 3 of treatment, among patients with an infectious virus at baseline, infectious SARS-CoV-2 was detected in 0.0% (n=0/92) of patients who received LAGEVRIO, compared with 21.8% (n=20/96) of patients who received placebo.
- At Day 5, infectious virus was detected in 0.0% (n=0/91) of patients in the LAGEVRIO arm compared with 2.2% (n=2/89) in the placebo arm.
- At Day 10, no infectious virus was detected in either arm for patients with an infectious virus at baseline.
- Molnupiravir was also associated with greater mean reductions from baseline in SARS-CoV-2 RNA than placebo from Days 3 through 10.
- However, molnupiravir and placebo were associated with comparable rates of viral RNA clearance through Day 29.
“In these exploratory analyses from our Phase 3 study in patients with mild to moderate COVID-19, LAGEVRIO cleared infectious SARS-CoV-2 faster than placebo among patients who had infectious virus at baseline, resulting in no infectious virus detected at Day 3, 5 or 10,” said Dr. Jay Grobler, associate vice president, infectious diseases and vaccines, Merck Research Laboratories, in a press release issued on April 1, 2022.
“These data reinforce our confidence in the potential of LAGEVRIO as a part of the solution to the COVID-19 pandemic.”
The Phase 3 MOVe-OUT clinical trial evaluated LAGEVRIO 800 mg twice-daily in non-hospitalized, unvaccinated adult patients with laboratory-confirmed mild to moderate COVID-19, symptom onset within five days of study randomization, and at least one risk factor associated with poor disease outcomes (heart disease, diabetes).
In addition to the MOVe-OUT trial, molnupiravir is being evaluated for post-exposure prophylaxis in MOVe-AHEAD, a Phase 3 study evaluating the efficacy and safety of molnupiravir in preventing the spread of COVID-19 within households.
In the U.S. and select markets, LAGEVRIO is the approved trademark for molnupiravir.
Note: The press release was edited for clarity and manually curated for mobile readers.

The peer-review New England Journal of Medicine journal recently published Original Article results showing early Ivermectin use in humans failed to reduce COVID-19 hospitalizations in patients in Brazil.
Announced on March 30, 2022, this phase 3 clinical trial was double-blinded and randomized, the gold standard for research.
A total of 1,358 patients were randomly assigned to receive ivermectin (679 patients) or a placebo (679).
Overall, 100 patients (14.7%) in the ivermectin group had a primary-outcome event, as compared with 111 (16.3%) in the placebo group (relative risk, 0.90; 95% Bayesian credible interval, 0.70 to 1.16).
Of the 211 primary-outcome events, 171 (81.0%) were hospital admissions.
The findings were similar to the primary analysis in a modified intention-to-treat analysis that included only patients who received at least one dose of ivermectin or placebo (relative risk, 0.89; 95% Bayesian credible interval, 0.69 to 1.15) and in a per-protocol analysis that included only patients who reported 100% adherence to the assigned regimen (relative risk, 0.94; 95% Bayesian credible interval, 0.67 to 1.35).
And there were no significant effects of ivermectin use on secondary outcomes or adverse events.
In conclusion, these researchers stated 'We did not find a significantly or clinically meaningful lower risk of medical admission to a hospital or prolonged emergency department observation (primary composite outcome) with ivermectin administered for three days at a dose of 400 μg per kilogram per day than with placebo.'
'We found no important treatment effects with ivermectin on the secondary outcomes.'
'The evidence supporting the role of ivermectin in the treatment of Covid-19 is inconsistent.'
The U.S. FDA previously approved ivermectin for people with intestinal strongyloidiasis and onchocerciasis, conditions caused by parasitic worms. In addition, topical forms of Ivermectin are approved to treat external parasites like head lice and skin conditions (rosacea).
Other COVID-19 treatments, authorized, experimental, and off-label are listed at PrecisionVaccinations.com/treatments.
Note: This NEJM article was edited for clarity and manually curated for mobile readers.

The U.S. Health and Human Services confirmed launching its own COVID-19 information portal on March 30, 2022. The new COVID.gov website's theme is 'Find COVID-19 guidance for your community.'
The White House says 'COVID.gov will provide one-stop shopping for all things COVID-19.'
'Among the site's features is a tool that lets people find out the infection status in their county.'
'Users can also find out where to get a vaccine, see locations that participate in the "test-to-treat" program where people who test positive can immediately get oral treatment, and locate places to get free masks.'
The government's new site also includes weblinks to order free at-home tests.
The primary advantage for consumers is the previously published U.S. CDC clinical information is more user-friendly and secure.
Note: Official websites use a '.gov' URL which indicates it belongs to an official government organization in the United States.
The U.S. Centers for Disease Control and Prevention (CDC) modified its Travel website on March 30, 2022, saying 'cruise ships may advise all passengers and crew that they do not have to wear a mask when outdoors.'
'Nor inside their cabin.'
And, 'they may also designate areas where masks are not required, such as casinos, bars, spas, entertainment venues, and dining areas as only accessible to passengers and crew who are fully COVID-19 vaccinated according to the cruise ship's policies.'
'Follow ship-specific mask protocols, which may change as the pandemic evolves and differ for passengers based on their vaccination status,' says the CDC website.
'Today's decision by the U.S. CDC to altogether remove the Travel Health Notice for cruising recognizes the effective public health measures in place on cruise ships and begins to level the playing field between cruise and similarly situated venues on land, for the first time since March 2020,' says a press statement issued by the Cruise Line International Association.
Additional cruise ship news is posted at Vax-Before-Travel.com/cruise.
Note: The CDC website post was edited for clarity and manually curated for mobile readers.

Pennsylvania-based Antios Therapeutics, Inc. today announced new data from the Phase 1b and 2a clinical trials of ATI-2173, its investigational proprietary drug candidate and the only Active Site Polymerase Inhibitor Nucleotide (ASPIN) in clinical development for hepatitis b virus (HBV).
These data showed that ATI-2173 alone or combined with tenofovir disoproxil fumarate (TDF) was generally well-tolerated among the cohorts.
And ATI-2173 and TDF suppressed HBV DNA and induced declines in biomarkers of cccDNA activity.
Data from these trials will be presented in poster sessions, which are highlighted below:
- Combining ATI-2173 with TDF, a chain-terminating nucleos(t)ide analogue, potently suppressed HBV DNA and induced declines in biomarkers of cccDNA activity.
- ATI-2173, as a monotherapy and in combination with TDF, decreased circulating HBV RNA with similar kinetics to HBV DNA.
- Patients receiving ATI-2173 and TDF combination treatment had normal alanine aminotransferase (ALT) levels or ALT levels less than 1x the upper limit of normal at the end of treatment, demonstrating the ability to administer this compound for up to 90 days.
- Many of the patients receiving ATI-2173 in combination with TDF were below the limit of quantification at the end of treatment.
- Successful targeting of ATI-2173 to the liver greatly decreased systemic exposure to clevudine in the blood, which may lead to enhanced safety and efficacy.
- ATI-2173 showed tolerability at 25 mg and 50 mg once-daily doses. Preliminary pharmacokinetic data analyses showed no drug-drug interaction between ATI-2173 and TDF. All subjects completed dosing without serious adverse events or adverse events leading to study drug discontinuation.
"As we continue to understand potential treatment options for the hepatitis B virus, these data are important and augment our understanding of the full potential effects of the combination on/off-treatment suppression," said Nancy Reau, M.D., Professor of Internal Medicine and Hepatology Section Head at Rush Medical College, in a press release issued on March 30, 2022.
"We are seeing potential clinical benefits in combination treatment."
For example, "in the Phase 2a study, no patients receiving ATI-2173 in combination with TDF had an ALT flare off-treatment, and every patient completed the combination treatment for up to 90 days."
Hepatitis B is a liver infection caused by HBV that can cause chronic infection, which leads to a higher risk of death from cirrhosis and liver cancer, says the U.S. CDC.
Approximately 900,000 people die every year from complications related to chronic HBV infection despite the availability of effective vaccines and current treatment options.
Hepatitis vaccine news is posted at PrecisionVaccinations.com/hepatitis.
Note: This news article edited the press statement for clarity and curated it for mobile readers.

The Janssen Pharmaceutical Companies of Johnson & Johnson recently announced the U.S. FDA approved CABENUVA for the treatment of HIV-1 in virologically suppressed adolescents who are 12 years of age or older, weigh at least 35 kg, and are on a stable antiretroviral regimen, with no history of treatment failure, nor known or suspected resistance to either cabotegravir or rilpivirine.
Co-developed as part of a collaboration with ViiV Healthcare, CABENUVA is the first and only complete long-acting HIV-1 treatment regimen and the first to be made available for eligible adolescents.
Announced on March 29, 2022, this news builds on Janssen’s ongoing commitment to fighting HIV and improving HIV treatment options for young people living with HIV.
This is an essential announcement since about 21% of all new HIV diagnoses in the U.S. and dependent areas in 2018 were by people aged 13-24.
“HIV remains one of the most significant challenges in global health, and as part of our decades-long commitment to fighting HIV, Janssen is working tirelessly to advance innovative new treatment options for young people living with HIV,” said James Merson, Ph.D., Global Therapeutic Area Head, Infectious Diseases, Janssen Research & Development, LLC, in a press release.
“With this milestone, we’re continuing to redefine how HIV can be managed so that even more people, including adolescents, can benefit from long-acting injectable therapies.”
CABENUVA is FDA Approved as a once-monthly or every-two-month treatment for HIV-1 in virologically suppressed adults and adolescents.
It contains ViiV Healthcare’s cabotegravir extended-release injectable suspension in a single-dose vial and rilpivirine extended-release injectable suspension in a single-dose vial, a product of Janssen Sciences Ireland Unlimited Company.
The once-monthly and every-two-months version of cabotegravir and rilpivirine injectable treatment has been approved for adults by the European Commission, Health Canada, the Australia Therapeutic Goods Administration, and the Swiss Agency for Therapeutic Products.
Regulatory reviews continue with additional submissions planned throughout 2022.
In addition, HIV vaccine development news is posted at PrecisionVaccines.com/HIV.
Note: The J&J press release was edited for clarity and curated for mobile readers.

Germany-based CureVac N.V. today announced that the first participant was dosed in a Phase 1 study of COVID-19 second-generation mRNA vaccine candidate, CV2CoV, which was developed in collaboration with GSK.
The dose-escalation study is being conducted at clinical sites in the U.S.
It intends to enroll up to 210 healthy adults, with data anticipated results in the second half of 2022.
"Continued innovation and progress in the development of mRNA-based vaccines is a critical prerequisite to combat the evolving COVID-19 pandemic and to further extend the possibilities of mRNA technology to a broad range of indications," said Dr. Klaus Edvardsen, Chief Development Officer of CureVac, in a press release issued on March 30, 2022.
"Our second-generation mRNA backbone was engineered to enable faster and stronger immune responses than our first-generation vaccine."
"This Phase 1 trial of CV2CoV will provide clinical data to further establish this backbone as a basis to flexibly address not only different COVID-19 variants, but also a range of other diseases and potential combination vaccines."
A preclinical study of CV2CoV in cynomolgus macaques, published in the journal Nature in November 2021, demonstrated rapid induction of higher antibody titers, better induction of immune memory, and stronger protective efficacy of CV2CoV compared to CureVac's first-generation vaccine candidate, CVnCoV.
The same study demonstrated comparable neutralizing antibody titers in vaccinated animals with either 12µg of CV2CoV or a 30µg standard dose of a licensed mRNA COVID-19 vaccine.
The CureVac-GSK infectious disease collaboration was first announced in July 2020.
Note: The CureVac media statement was edited for clarity and curated for mobile readers.

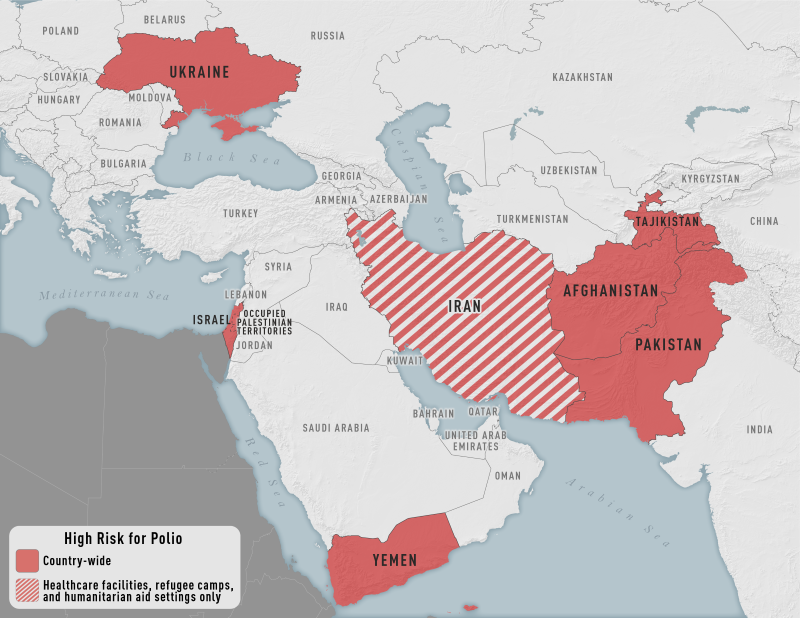
The U.S. Centers for Disease Control and Prevention (CDC) recently updated its polio vaccination recommendation for various countries in Africa, Asia, and Eastern Europe.
The CDC reissued Level 2, Practice Enhanced Precautions, travel alerts on March 23, 2022, that say 'Some international destinations are considered high risk for polio.'
'Before traveling to any high-risk destination, the CDC recommends that adults who previously completed the full, routine polio vaccine series receive a single, lifetime booster dose.'
'Even those who have been sick with polio previously may need a booster dose of polio vaccine.'
The CDC recommends administering a single, lifetime booster dose of inactivated poliovirus vaccine (IPV) to adult travelers that meet certain conditions, which are as follows:
- Are going to destinations considered high risk for polio,
- Have previously completed the full, routine polio vaccine series, and
- Have not already received an adult booster dose.
While polio is a crippling and potentially deadly disease that affects the nervous system, says the CDC.
However, most people only have minor symptoms, such as fever, tiredness, nausea, headache, nasal congestion, sore throat, cough, stiffness in the neck and back, and pain in the arms and legs.
But in rare cases, polio infection causes permanent loss of muscle function (paralysis).
And polio can be fatal if the muscles used for breathing are paralyzed or if there is an infection of the brain.
An extended listing of polio vaccines, including the IPV, is posted at Vax-Before-Travel.com/polio.
Furthermore, travel vaccination appointments can be requested at this weblink.
Note: These CDC alerts were edited for clarity and curated for mobile readers.
Germany-based BioNTech SE today reported financial results for the full year ended December 31, 2021, and provided an update on its corporate progress and COVID-19 revenues.
BioNTech's share of collaboration partners' gross profit is based on COVID-19 vaccine sales in New York-based Pfizer Inc. and China-based Fosun Pharma's territories and represents a net figure.
- Full-year revenues of €18,976.7 billion (US$21.1 billion) and net income of €10,292.5 billion (US$11.1),
- Reiterate BioNTech COVID-19 vaccine revenue guidance of €13 billion to €17 billion for 2022,
- Approximately 2.6 billion doses of COMIRNATY®/BNT162b2 delivered to more than 165 countries and regions worldwide in 2021,
- Signed orders for 2022 delivery increased to 2.4 billion COVID-19 vaccine doses,
- Initiated expansion of Phase 3 clinical trials to include Omicron-based vaccine candidates,
- Focused on driving further transformation in 2022 by reinvesting COVID-19 vaccine profits to accelerate oncology and infectious disease programs, broaden mRNA pipeline, and scale-up business,
- Expanded clinical-stage oncology pipeline to 16 clinical programs with the initiation of nine clinical trials, including four randomized Phase 2 trials.
"Looking back, 2021 was an exceptional year during which BioNTech had a momentous impact on human health and the global economy with our first approved vaccine based on our mRNA technology," said Ugur Sahin, M.D., CEO and Co-Founder of BioNTech, in a press release issued on March 30, 2022.
"To continue our industry leadership, we intend to build on our 2021 success and rapidly advance multiple programs, including our mRNA-based immunotherapies, cell therapies, and bi-specific antibodies."
"At the same time, we are investing in our second-growth pillar, infectious diseases, and intend to advance our influenza and shingles vaccine candidates together with our partner Pfizer."
"In parallel, we also intend to invest heavily in regenerative medicine and autoimmune diseases with the aim to develop further therapeutic innovations addressing the high unmet medical need."
"Our core vision remains the foundation for all our activities: harnessing the power of the immune system to improve the health and lives of billions of people worldwide."
Note: The BioNTech statement was edited for clarity and curated for mobile readers.