Search API
Approximately 30% of children exposed to the Zika virus before birth were diagnosed with neurological impairments or microcephaly during their first years of life.
Approximately 2.6% of children born to Zika-infected mothers had microcephaly at birth or when first assessed, rising to 4% across the early preschool years.
The new research published in The Lancet Regional Health – Americas is based on an analysis of 13 studies conducted from 2015 to 2017 in Brazil, providing the clearest evidence of the scale of the impact associated with Zika infection during an expecting mother's pregnancy.
"The findings underscore the continued need to develop a safe and effective vaccine for preventing Zika virus infections during pregnancy," said Dr. Elizabeth Brickley, Associate Professor of Epidemiology at the London School of Hygiene and Tropical Medicine and a co-author of the study.
"While gaps in population-level immunity to the virus persist, the threat of a Zika virus re-emergence remains a concern for public health," wrote GAVI on December 2, 2022.
In 2022, 31,453 Zika cases were reported in the Region of the Americas.
As of December 5, 2022, the U.S. FDA has not approved a Zika vaccine candidate.

To better address the evolving risk of type 2 circulating vaccine-derived poliovirus (cVDPV2), the Global Polio Eradication Initiative (GPEI) with countries are deploying an innovative novel oral polio vaccine type 2 (nOPV2) to reduce the spreading of poliovirus.
The GPEI recently reported about 525 million doses of the nOPV2 polio vaccine had been administered across 25 countries under its WHO Emergency Use Listing.
As of November 30, 2022, an additional 15 countries have met the requirements for nOPV2 use in the event of an outbreak.
The new vaccine is a modified version of the type 2 monovalent Oral Polio Vaccine, which clinical trials have shown provides comparable protection against poliovirus while being more genetically stable and less likely to be associated with the emergence of cVDPV2.
This means that nOPV2 has the potential to be a significant tool to help stop polio outbreaks, says the GPEI.
In 2022, poliovirus was confirmed in non-endemic countries in Africa, Asia, Europe, India, Israel, the United Kingdom, and the state of New York.
To notify people of their potential polio health risks, the U.S. CDC issued an updated global Alert - Level 2, Practice Enhanced Precautions on November 30, 2022.
As of December 5, 2022, the nOPV2 vaccine is not approved by the U.S. FDA.
The IPV vaccine has been offered in the U.S. since 2022.
Additional polio outbreak information is posted at PrecisionVaccinations.com/Polio.

Zika virus (ZIKV) and dengue virus (DENV) are arthropod-borne pathogenic flaviviruses that co-circulate in many countries.
While two dengue vaccines are available as of December 5, 2022, the U.S. FDA has not approved any Zika vaccine candidate.
To understand some of the pressures that influence ZIKV evolution, researchers recently mimicked the natural transmission cycle by repeating serial passaging of ZIKV through cultured mosquito cells and either DENV-naive or DENV-immune mice.
Compared with wild-type ZIKV, the strains passaged under both conditions exhibit increased pathogenesis in DENV-immune mice.
This study's data indicate that ZIKV strains with enhanced transmissibility and pathogenicity can emerge in DENV-naive or -immune settings and that NS2B-I39 mutants may represent ZIKV variants of interest.
Zika vaccine candidate news is posted at ZikaNews.com/Vaccines.

A specialty vaccine company based in France announced today positive antibody persistence data one year after vaccination with a single dose of its chikungunya vaccine candidate, VLA1553.
Valneva SE's VLA1553 vaccine is currently on track to complete the rolling submission for Biologics License Application with U.S. Food and Drug Administration by the end of 2022.
This is important news since the FDA has not approved a chikungunya vaccine candidate as of December 5, 2022.
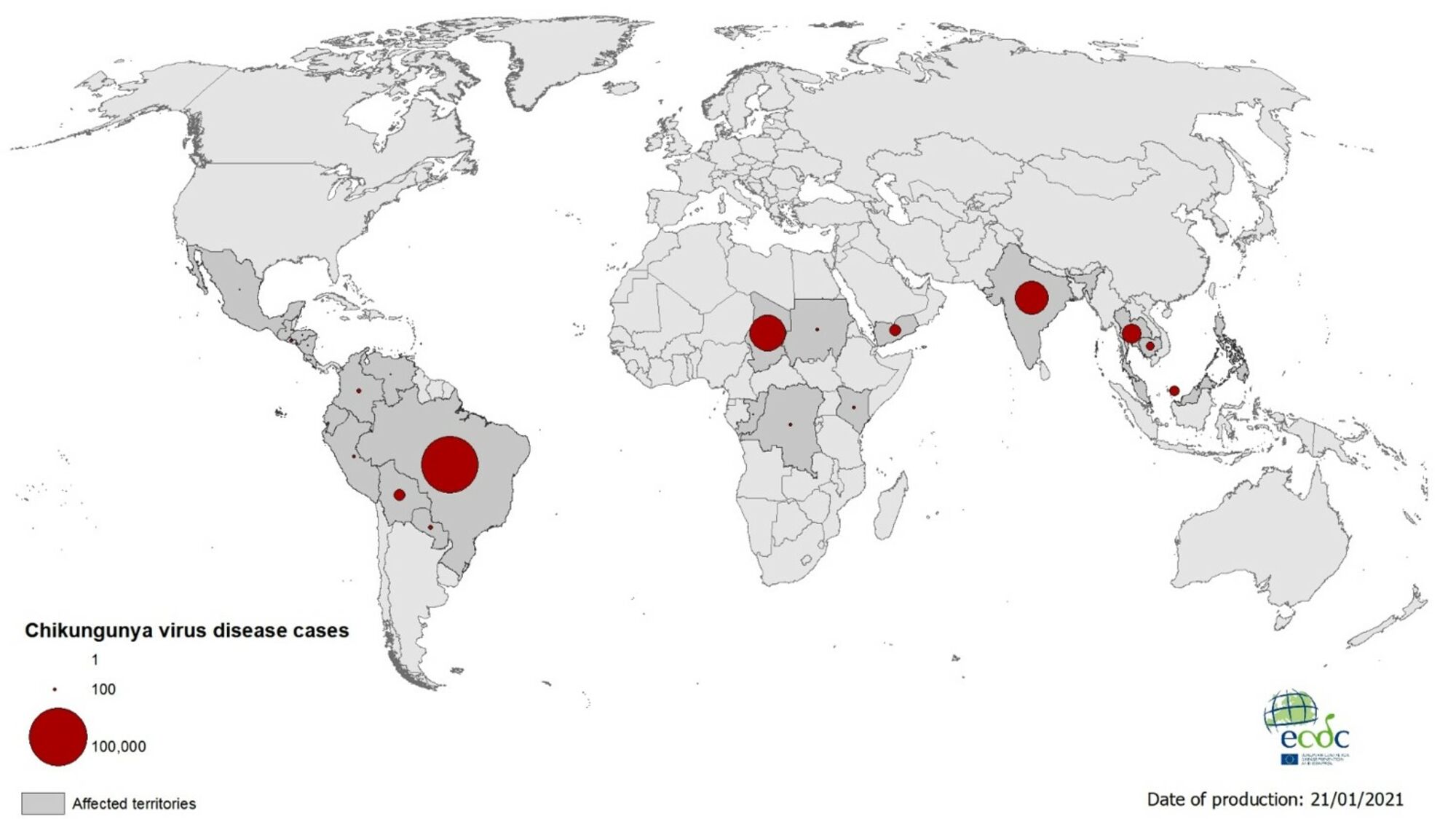
Chikungunya is a mosquito-borne viral disease that often causes sudden large outbreaks with high attack rates, affecting up to three-quarters of the population in areas where the virus is circulating.
An infection leads to symptomatic disease in up to 97% of people after three to seven days following the mosquito bite.
The clinical symptoms include acute onset of fever, debilitating joint and muscle pain, headache, nausea, rash, and chronic arthralgia, says the U.S. CDC.
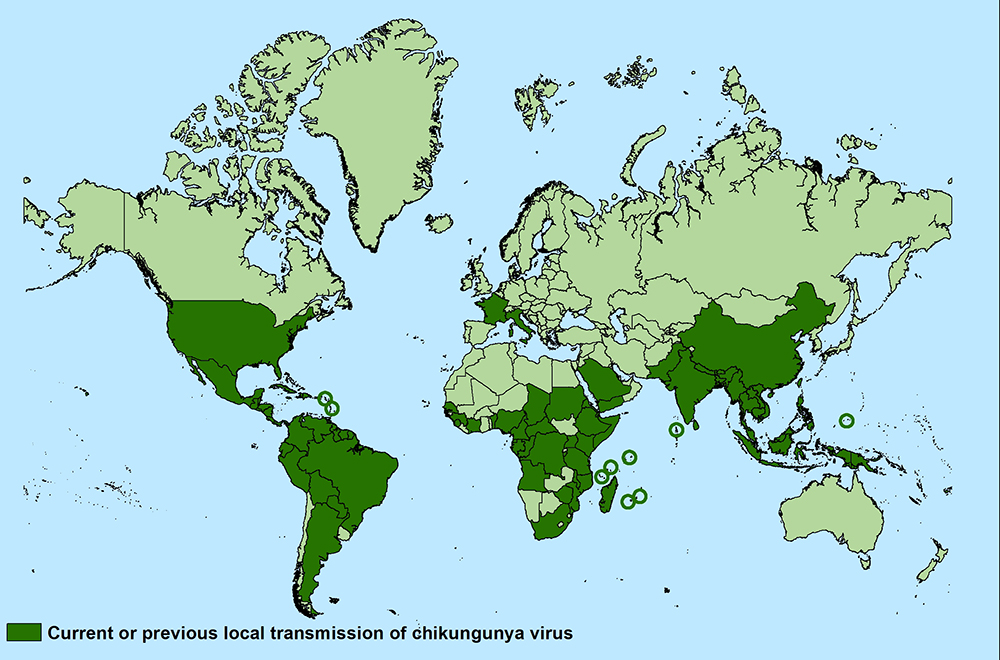
In late 2013, the first local transmission of the chikungunya virus in the Americas was identified in Caribbean countries and territories. As of September 2020, there were more than 3 million reported cases in the Americas.
High-risk areas of infection are places where chikungunya virus-carrying mosquitos are currently endemic, including the Americas, parts of Africa, and Southeast Asia.
Most cases in the U.S. are travel-related, not locally acquired.
Additional chikungunya vaccine candidate news is posted at PrecisionVaccinations.
Valneva SE today reported positive antibody persistence data twelve months after vaccination with a single dose of its chikungunya vaccine candidate, VLA1553.
Furthermore, antibody levels remained stable between month 6 to month 12, and there were no safety concerns identified during follow-up, confirming VLA1553's safety profile observed in earlier studies.
This finding is essential since the U.S. FDA has not approved any chikungunya vaccine candidate as of December 5, 2022.
Chikungunya is a mosquito-borne viral disease that often causes sudden large outbreaks with high attack rates, affecting about one-third of the population in areas where the virus is circulating, says the U.S. CDC.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release on December 5, 2022, "We are excited about these twelve-month data which are in line with what we saw from our previous read out at month six, and strengthen the possibilities of inducing a long-lasting antibody response with our chikungunya vaccine candidate."
"We look forward to completing the BLA rolling submission to the FDA and potentially changing people's lives."
"If our investigational vaccine is approved, we are confident it can help address this growing public health threat."
Valneva expects to finalize its BLA submission with the FDA by the end of 2022.
Once completed, and if the FDA accepts the filing, the FDA will determine priority review eligibility along with the action due date upon which it will complete its evaluation.
Valneva also initiated a Phase 3 trial in adolescents conducted in Brazil by Instituto Butantan to support the label extension in this age group following a potential initial regulatory approval.
Additional chikungunya vaccine candidate news is posted at PrecisionVaccinations.com/Chilungunya.
The Health Department of the City of Columbus, Ohio, recently released data that confirmed fifty unvaccinated children had been infected with the measles virus since June 2022.
Of these measles cases, 52% of these patients were under two years of age.
As of December 2, 2022, Columbus, Franklin County Public Health, and Ross County Health District reported 20 children had been hospitalized.
The City of Columbus Health Commissioner Dr. Mysheika Roberts recently said during a news conference: "No matter what your age, if you're an adult not vaccinated, take a measles-mumps-rubella (MMR) vaccine."
"If you're a child and eligible to get vaccinated, get the MMR," reported The Columbus Dispatch.
Measles is very contagious and can be serious. An unvaccinated child can get measles when traveling abroad or even in the U.S., says the U.S. CDC.
Measles spreads through the air when an infected person coughs or sneezes. It is so contagious that if one person has it, up to 90% of people in the area could become infected if they are not protected.
Various MMR vaccines are available at most clinics and pharmacies in the U.S.
Additional measles outbreak 2022 news is posted at PrecisionVaccinations.com/MeaslesOutbreak.
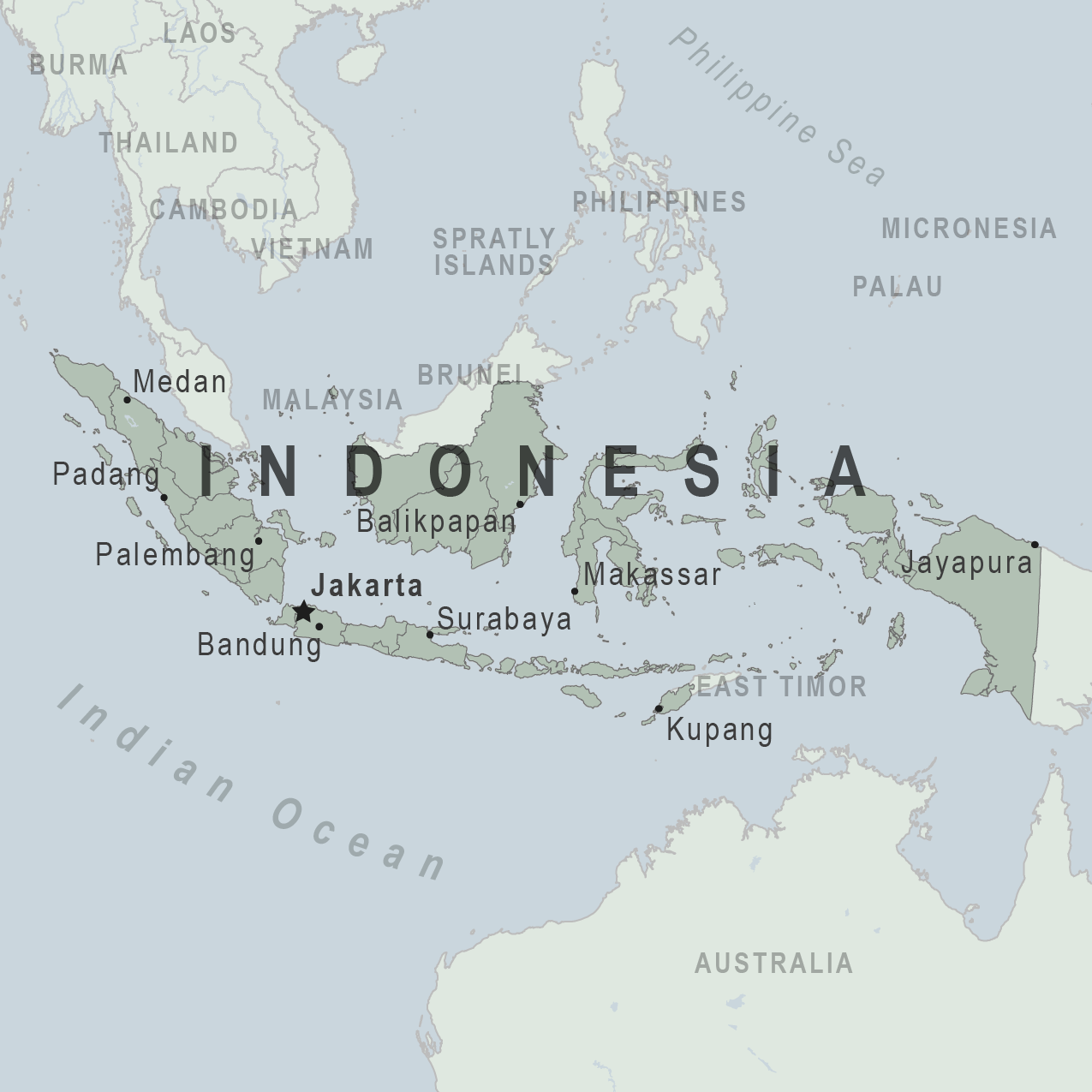
According to the National Disaster Management Agency reporting, Indonesia's Mount Semeru 12,000-foot tall volcano, recently erupted, causing smoke plums to rise high into the sky.
Local media reported volcanic ash was seen rising high and tending to the south.
Increased activities of the volcano on December 4, 2022, prompted authorities to widen the danger zone to 8 kilometers (5 miles) from the crater, reported NPR. Semeru is located southeast of the capital Jakarta.
The Center for Volcanology and Geological Hazard Mitigation has raised the status of Mount Semeru from level III to level IV.
The Emergency Contact number for American citizens at the U.S. Embassy Jakarta, please call +62-21-5083-1000, and press 1 for assistance.
And Americas currently in this area of Indonesia are encouraged to enroll in the State Department's Smart Traveler program to receive critical information from the Embassy about safety conditions and empower visitors to make informed decisions about their travel plans.
The actual impact of this volcano eruption on international flights remains to be confirmed.
Semeru, also known as Mahameru, has erupted numerous times in the last 200 years.
Semeru's last major eruption was in December 2021, when over 50 related fatalities and the evacuation of more than 10,000 people were confirmed.
Indonesia is an archipelago of more than 270 million people, situated along the Pacific's Ring of Fire, and is prone to earthquakes and volcanic activity.
The number of international visitor arrivals in Indonesia steadily from 2012 to 2019, reaching about 16 million, reports Statista. But, unfortunately, the recent pandemic significantly reduced to just 1.6 million in 2021.
Prospective visitors can learn about health issues in Indonesia by visiting the U.S. CDC.
Indonesia was included in the CDC's Global Alert - Level 2, Practice Enhanced Precautions, regarding polio outbreaks.
The scientific community is still learning about the Zika virus. In 2022, the Pan American Health Organization (PAHO) reported nearly 30,000 cases throughout the Americas and four deaths.
Furthermore, the PAHO says more than 500 million people are living in areas at risk of Zika, Dengue, or Chikungunya, because of the presence of the Aedes Aegypti mosquito.
As of October 2022, Brazil has the highest cumulative incidence.
By the end of 2016, 48 countries and territories in the Americas had reported 175,063 confirmed Zika patients.
In addition, 22 countries and territories reported 2,439 cases of a congenital syndrome (Microcephaly) associated with Zika.
The U.S. CDC recently stated because the mosquitoes that spread Zika are found throughout Puerto Rico, people living on the island who have not already been infected are at risk for infection.
Although the Zika virus is now circulating at low levels across the region, the 2016 outbreak had a lasting impact: the babies born with microcephaly during the 2015-2016 outbreak are now turning 6 and 7 years old.
Their developmental challenges continue as parents and health authorities grapple with their condition.
"It is important not to let our guard down. Surveillance efforts must be maintained to ensure we can respond quickly when the virus is detected," said Dr. Marcos Espinal, PAHO's interim Assistant Director, in a media release.
PAHO continues to help countries develop and maintain the ability to detect and confirm cases, treat people and implement effective strategies to reduce the presence of mosquitoes.
"The outbreak was a complicated chess game," said Dr. Sylvain Aldighieri, the PAHO Incident Manager at the time of the Zika epidemic, "with many moving parts, all of which were constantly evolving, making it much more challenging for scientists to study and understand."
As of December 4, 2022, there is no U.S. FDA-approved medicine or vaccine to prevent a Zika infection.
Although there are some promising vaccine candidates in the pipeline, the best prevention is still avoiding mosquito bites, says the PAHO.
Additional news is posted at ZikaNews.com.


