Cholera Outbreaks
Cholera Outbreaks September 2025
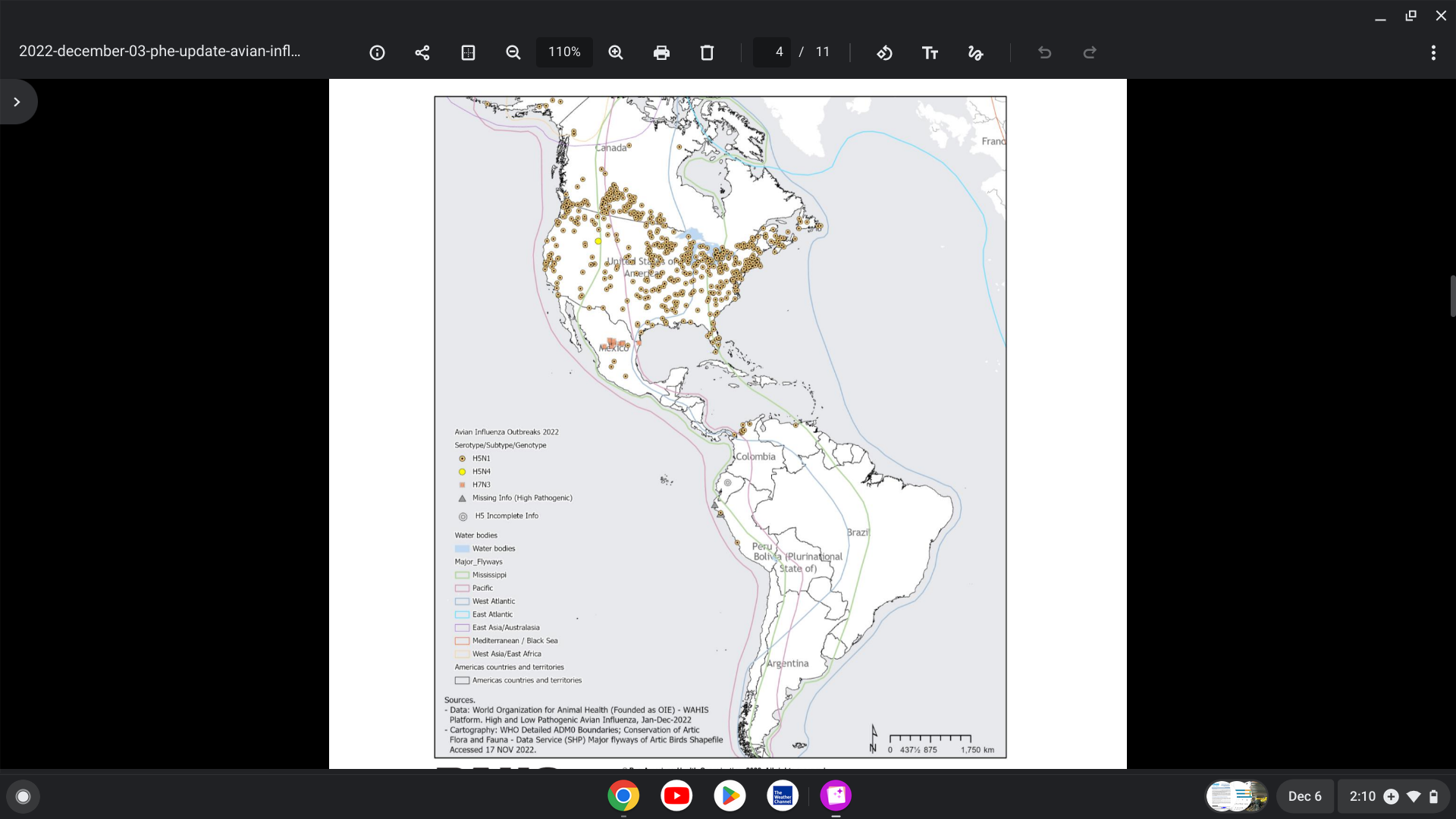
The World Health Organization (WHO) has recorded seven cholera pandemics over the past two centuries. The current (7th) cholera epidemic is considered to have started in 1961. As of September 2025, over 409,222 cholera/Acute Watery Diarrhoea cases and 4,738 deaths were reported globally, from 31 countries, with six of the 31 countries reporting case fatality rates above 1%. The WHO's Global Cholera and Acute Watery Diarrhoea Dashboard was updated in 2025. In 2024, 804,721 cholera cases and 5,805 related fatalities were reported from 33 countries. Data show that 472,697 cholera cases were reported to the WHO in 2022, up from 223,370 in 2021.
The U.S. Centers for Disease Control and Prevention (CDC) has identified an unprecedented global increase in cholera outbreaks over the last four years: 2022, 2023, 2024, and 2025. In addition, the Pan American Health Organization (PAHO) and the European CDC, and the WHO have reported active cholera transmissions in Angola, Benin, Cameroon, the Democratic Republic of the Congo, Eswatini, Ethiopia, India, Haiti, Kenya, Malawi, Mozambique, Myanmar, Nepal, Niger, Nigeria, Somalia, South Africa, South Sudan, Tanzania, Uganda, and Zambia, and Syria.
Cholera Cases in the U.S.
With modern water systems, there are now typically fewer than 20 cholera cases a year. Nearly all cholera cases reported in the U.S. are acquired during international travel. As of May 2025, there were (1) cholera cases reported in the U.S. As of December 31, 20224, week #52, eight cholera cases were reported to the CDC this year. In 2023, there were 17 cases, and in 2022, the CDC confirmed that eight travelers infected with cholera arrived from Pakistan, Iraq, and Bangladesh. Additionally, the CDC's Clinician Outreach and Communication Alert in 2023 reported an unprecedented global increase in cholera infections.
Cholera Vaccines
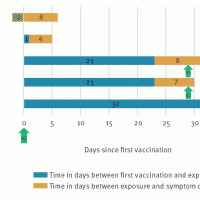
As of September 2025, various cholera vaccines have been approved by the WHO but remain in limited availability globally.