Search API
Peru's Culture Ministry website recently posted a notice that it had closed the country's most famous tourist attraction "to protect the safety of tourists and the population in general."
As of January 21, 2023, the 15th-century ancient ruins of Machu Picchu were not accepting future visitors to this mountaintop site, as the train service was recently closed.
There are a number of hiking routes along the Inca Trail to reach Machu Picchu, however, it takes about five days to reach the top.
Traditionally, over 2,000 people visit Machu Picchu daily.
Machu Picchu is located in the Eastern Cordillera of southern Peru on a 2,430-meter mountain ridge.
Cusco, Peru, where Machu Picchu is located, has been the site of some civil unrest in 2023.
The local airport, Alejandro Velasco Astete, was closed recently.
But, flights are expected to resume at Arequipa's Alfredo Rodriguez Ballon International Airport on January 26, 2023. According to the U.S. Embassy Peru, U.S. citizens should contact the appropriate airline for flight rescheduling.
For emergencies involving American citizens in Peru, please email [email protected] or call +51-1-618-2000. And U.S. citizens are advised to register in STEP to receive alerts and messages from the U.S. Embassy in Lima.

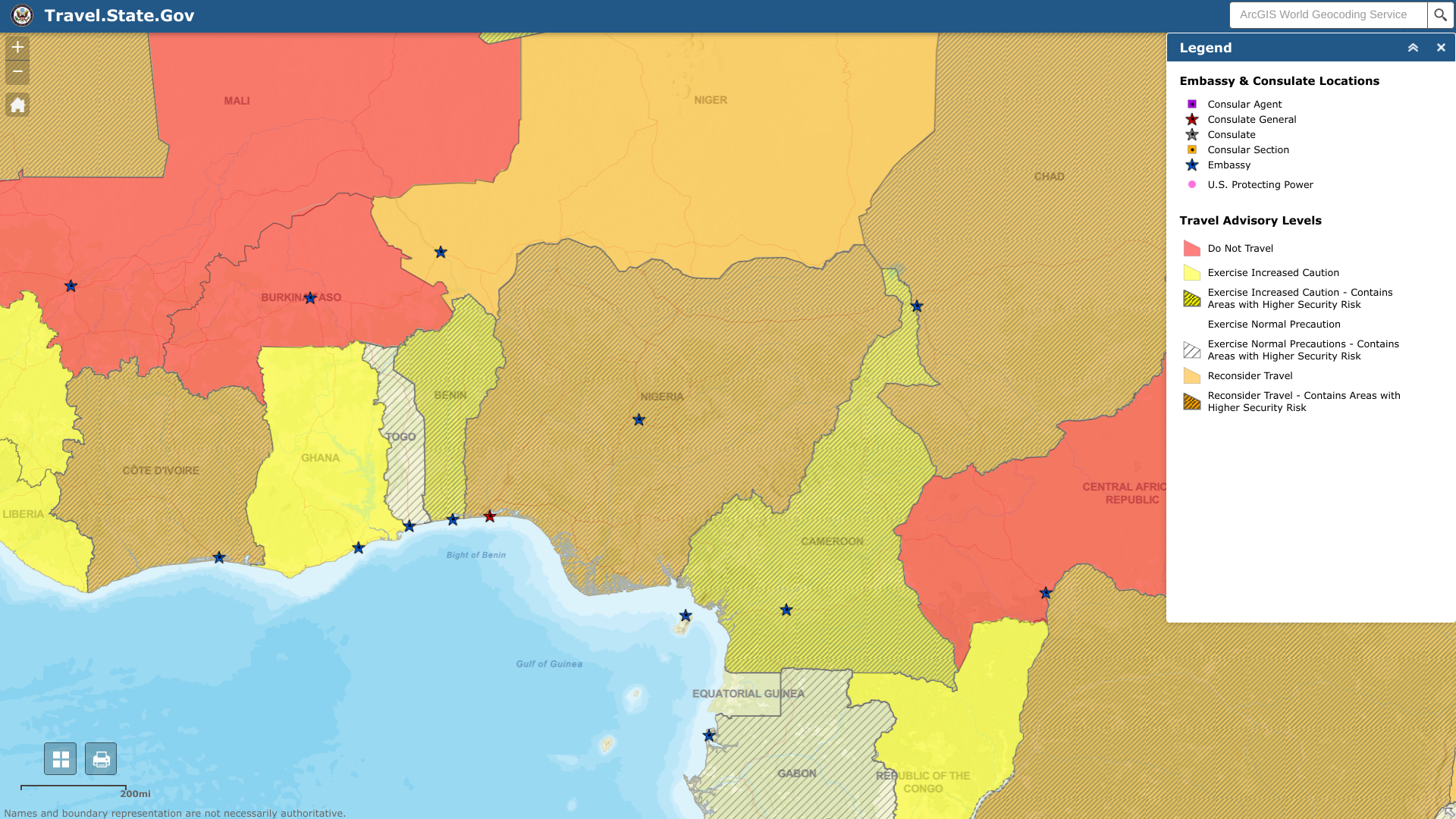
The U.S. Department of State updated its Level 3 Travel Advisory for the federal republic of Nigeria, located in west Africa.
Local media reported people should exercise caution around events in Lagos State from Jan. 23-24, 2023. And plan for localized transport disruptions.
As of January 23, 2023, the State Department says Do Not Travel to certain areas due to civil unrest:
- Borno, Yobe, Kogi, and northern Adamawa states,
- Bauchi, Gombe, Kaduna, Kano, Katsina, and Zamfara states,
- Coastal areas of Akwa Ibom, Bayelsa, Cross River, Delta, and Rivers states (except Port Harcourt).
Furthermore, the U.S. Consulate in Lagos provides all routine and emergency services to U.S. citizens in Nigeria. The U.S. Embassy Abuja can only provide emergency assistance to U.S. citizens in Abuja.
U.S. citizens in Nigeria who require assistance should contact [email protected] or +234 1 460 3410.
Additionally, they should enroll in the Smart Traveler Program to receive alerts and facilitate extraction during emergencies.
From a health perspective, the U.S. Centers for Disease Control and Prevention (CDC) advises future Nigeria visitors to speak with a travel vaccine advisory about one month before departure.
The CDC has recently issued various Travel Health notices for Nigeria's disease outbreaks, such as yellow fever, polio, measles, and Mpox.
The JAMA Network Open recently published an Original Investigation that found 41% of adults with impaired immune systems had received a 4th mRNA vaccine dose.
However, only 1% had received their recommended 5th dose
'Our results highlight a substantial gap in adherence to recommendations for mRNA monovalent COVID-19 booster doses,' wrote these researchers on January 20, 2023.
"Given the increased risk for severe COVID-19 in this vulnerable population and the well-established additional protection afforded by booster doses, targeted and tailored efforts to ensure that immunocompromised individuals remain up to date with COVID-19 booster dose recommendations are warranted," the researchers added.
Immunocompromised individuals (i.e., persons with immunocompromising conditions or who are taking immunosuppressive medications) often mount weaker immune responses to vaccines and experience higher rates of vaccine failure compared with immunocompetent individuals.
As of January 2023, both the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention have progressively amended COVID-19 vaccine authorizations and recommendations to include additional COVID-19 vaccine doses for immunocompromised individuals.
Disclosures: Pfizer, a company producing mRNA vaccines, sponsored his study. Corresponding Author: Sara Y. Tartof, Ph.D., MPH, Department of Research and Evaluation, Kaiser Permanente Southern California ([email protected]).

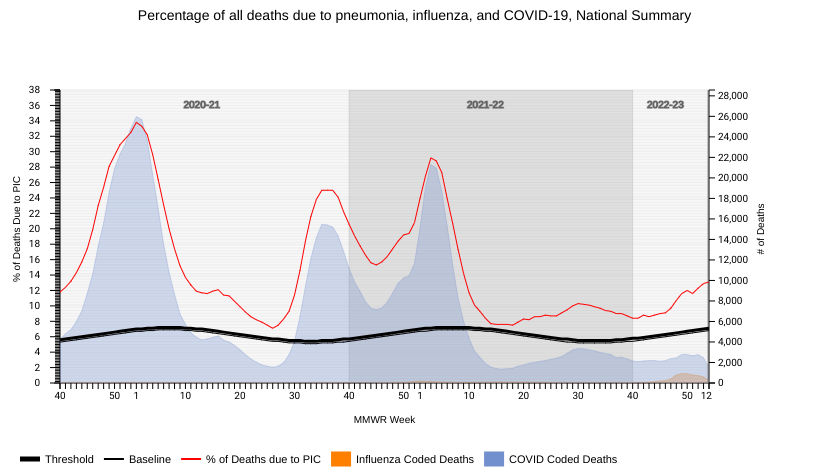
The U.S. Centers for Disease Control and Prevention (CDC) published Key Updates for Week #2, ending January 14, 2023. This CDC report highlights both good and unfortunate news.
The Weekly U.S. Influenza Surveillance Report says seasonal influenza activity continues to decline across the U.S., with three regions below their outpatient respiratory illness baselines for the first time since October 2022.
And the majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this season’s influenza vaccine, which remain available at most clinics and pharmacies in the U.S.
Furthermore, the National Center for Health Statistics Mortality Surveillance data available on January 19, 2023 shows that overall flu-related fatalities have decreased for the past four weeks.
During week #2, there were 2,954 pneumonia, influenza, and/or COVID-19 (PIC) deaths.
Among those PIC deaths, 1,422 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, 1,281 documented pneumonia, and 251 listed influenza.
Unfortunately, the CDC also confirmed six additional influenza-associated pediatric fatalities have occurred during the 2022-23 flu season. This news increases the total of 85 pediatric flu deaths reported so far this season.
During the last flu season, there were only 45 pediatric fatalities related to the flu.
The CDC says an annual flu shot remains the best way to protect against influenza infections and can also prevent serious outcomes in people who get vaccinated but still get sick with the flu.
CDC recommends that everyone ages six months and older get an annual flu vaccine as long as flu activity continues, which could be several additional months.
So far this flu season, 171.52 million doses have been distributed in the U.S.
HUTCHMED Limited today announced it entered into an exclusive license agreement with a subsidiary of Takeda Pharmaceutical Company Limited to further the global development, commercialization, and manufacture of fruquintinib.
Fruquintinib is orally administered and has the potential to be used across subtypes of metastatic colorectal cancer (“CRC”), regardless of biomarker status.
It is a highly selective and potent inhibitor of vascular endothelial growth factor receptors -1, -2, and -3.
CRC is a type of cancer that starts in either the colon or rectum.
Although early-stage CRC can be surgically resected, metastatic CRC remains an area of high unmet need with poor outcomes and limited treatment options.
HUTCHMED confirmed on January 23, 2023, it will receive up to US$1.13 billion, including US$400 million upfront on closing, as well as potential regulatory, development, and commercial sales milestone payments, plus royalties on net sales.
“Fruquintinib has the potential to change the treatment landscape for patients with refractory metastatic CRC who need additional treatment options. We look forward to utilizing our development and commercial capabilities to expand the potential of this innovative medicine to patients beyond China,” commented Teresa Bitetti, President of the Global Oncology Business Unit at Takeda, in a related press release.
Positive results of FRESCO-2, the global Phase III multi-regional clinical trial of fruquintinib in refractory metastatic CRC, were presented at the European Society for Medical Oncology Congress in September 2022. FRESCO-2 met its primary endpoint of improving overall survival in patients with metastatic CRC and was generally well tolerated.
According to the International Agency for Research on Cancer, CRC is the third most prevalent cancer worldwide, associated with more than 935,000 deaths in 2020.
In the U.S., an estimated 155,000 patients were diagnosed with CRC, and there were 54,000 related fatalities.
HUTCHMED stated it would continue to focus on progressing late-stage clinical trials and the commercialization of fruquintinib in mainland China in collaboration with Eli Lilly and Company, where it is approved under the brand name ELUNATE® for the treatment of patients with metastatic CRC who have been previously treated with fluoropyrimidine, oxaliplatin, and irinotecan, including those who have previously received anti-vascular endothelial growth factor therapy and/or anti-epidermal growth factor receptor therapy (RAS wild type).
ELUNATE has been included in the China National Reimbursement Drug List since January 2020 and was commercially launched in China in November 2018.

Genexine recently announced it received Fast Track Designation from the Korean Ministry of Food and Drug Safety (MFDS) for GX-188E, its first-in-class proprietary therapeutic DNA vaccine targeting advanced cervical cancer.
Following an evaluation of Phase 2 data from the recently completed clinical trial in advanced cervical cancer, Korea’s Health Authority (MFDS) concluded that GX-188E met the criteria for fast-track designation.
Genexine recently reported Phase 2 trial data which evaluated the efficacy and safety of the combination of GX-188E and KEYTRUDA®, anti-PD-1 therapy, in a total of 65 patients with HPV 16- and/or HPV 18- positive recurrent or metastatic advanced cervical cancer.
The final efficacy analysis evaluated in 60 patients showed an Objective Response Rate of 35%, indicating that of the 60 patients with advanced cervical cancer, 21 patients saw either over 30% reduction in tumor size or complete remission.
“We are grateful to the MFDS for their careful evaluation and recognition that GX-188E has the potential to be a key life-saving drug for the treatment of advanced cervical cancer,” said Neil Warma, Genexine’s President and CEO, in a press release on January 20, 2023.
“We are committed to the cancer patients in which this therapy could be effective..... We are designing the optimal Phase 3 study with GX-188E and expect to initiate that study this year (2023).”
HPV prevention vaccines are generally available at clinics and pharmacies worldwide.

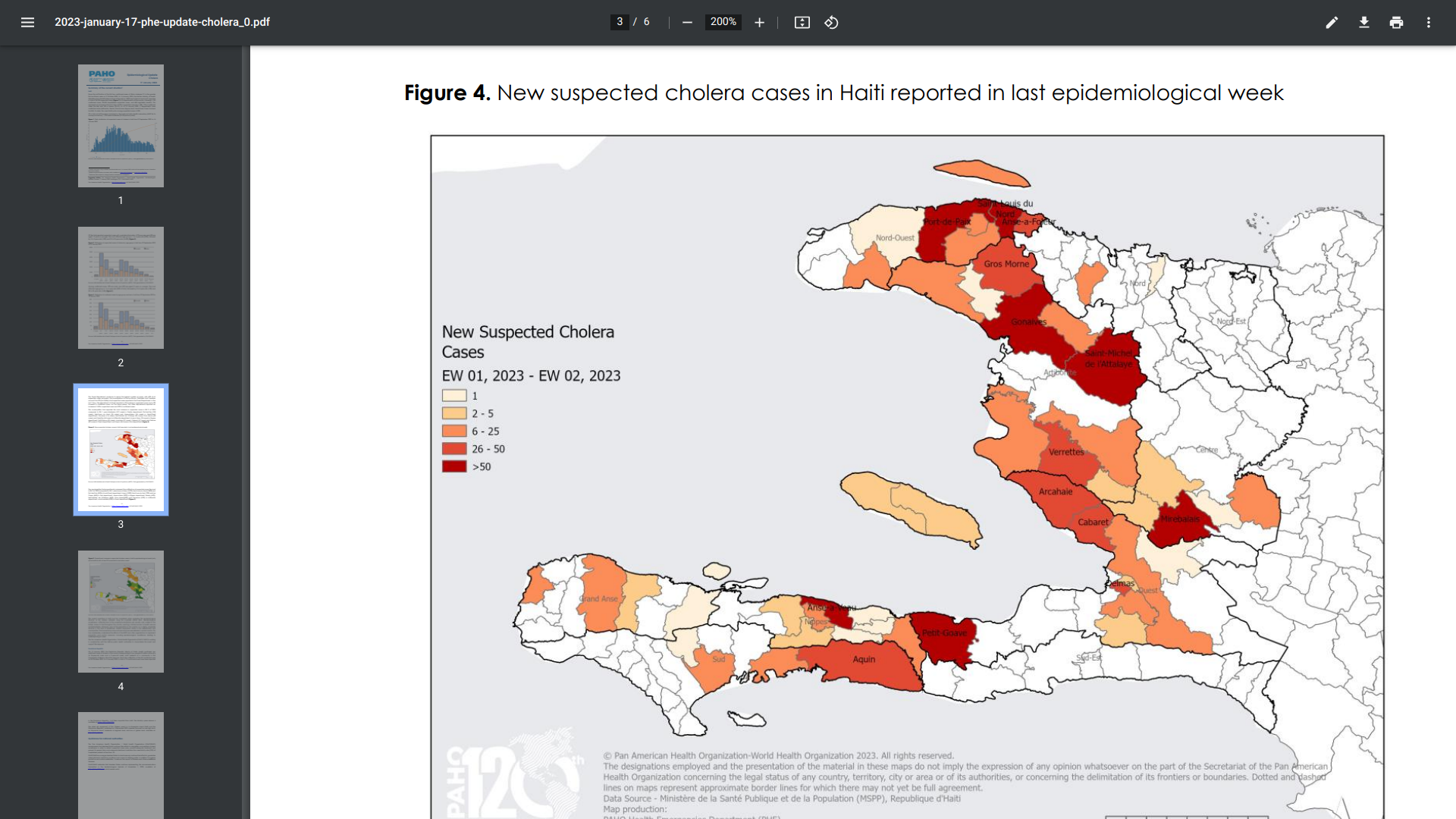
The Pan American Health Organization (PAHO) recently reported that since the initial cholera cases (Vibrio cholerae O1) in the greater Port-au-Prince area in October 2022, the Haitian Ministry of Health has reported a total of 24,232 suspected cases and 483 registered fatalities.
As of January 14, 2023, the Ouest Department, which includes the municipalities of Port-au-Prince, Cité-Soleil, and Carrefour, continues to report the highest number of cases, with 67% (N=10,836) of all suspected cases reported.
During a similar time frame, a total of 19 confirmed cases have been reported in the Dominican Republic (DR), with five of them imported from Haiti.
In a press release on January 15, 2023, the DR's Ministry of Public Health urged residents not to be alarmed and to remain attentive to the issued reports.
The latest PAHO risk assessment of the Cholera event in La Hispaniola Island (Haiti and the Dominican Republic) assesses the event as very high risk locally, moderate at the regional level, and low at the global level.
The U.S. Centers for Disease Control and Prevention (CDC) stated in 2022, vaccination may be considered for children and adults traveling to areas of active cholera transmission.
As of January 2023, cholera vaccines remain unavailable in the U.S.
Cholera is rare in travelers but can be severe. Certain factors may increase the risk of getting cholera or having severe disease. The CDC says avoiding unsafe food and water and washing your hands can also help prevent cholera.
Furthermore, the U.S. Department of Stated announced in December 2022 do not travel to Haiti due to civil unrest. U.S. citizens should depart Haiti now in light of the current security and health situation and infrastructure challenges.
Furthermore, clinicians should be prepared to treat cholera cases in travelers returning to the U.S. in 2023.