Search API
The Canadian government recently confirmed visitors to Buenos Aires, Argentina, should exercise high caution due to civil unrest.
As of January 31, 2023, Canada stated when in the Buenos Aires tourist areas of La Boca, Congreso, Florida Street, the Retiro bus station area, and San Telmo, be aware of your surroundings.
And in La Boca, always remain on Caminito Street, as thefts often occur on neighboring streets, and avoid the area after dark.
Furthermore, since 2019, several incidents of tourists have been followed from Buenos Aires' Ministro Pistarini International Airport to their hotels and robbed. In some cases, the criminals responded violently when the victims resisted.
If you are the victim of a crime, call the local police at 911, as the U.S. Embassy cannot take custody of U.S. citizens or intervene in Argentine legal procedures. All U.S. citizens are subject to the laws of the Government of Argentina.
The Embassy suggests enrolling in the STEP program to receive important information about safety conditions, helping you make informed travel plans.
The U.S. Embassy in Buenos Aires is located at Av. Colombia 4300 (C1425GMN). Telephone: (54-11) 5777-4533.
From a health perspective, the U.S. Centers for Disease Control and Prevention (CDC) says there are no travel health notices in effect for Argentina as of February 5, 2023.
However, the CDC suggests various travel vaccinations, including yellow fever.
Yellow fever vaccination with either Stamaril or YF-Vax is recommended for travelers ≥9 months of age going to Corrientes and Misiones Provinces. And is not recommended for travelers going to Formosa Province and designated areas of Chaco, Jujuy, and Salta Provinces.
And the U.K.'s travel advisory for Argentina includes notices regarding dengue and Zika virus transmission.

Public Health recently announced it was extending negative COVID-19 test requirements for arriving air travelers from the Peoples' Republic of China.
Initially announced in early January 2023, this extension is scheduled to be a requirement until April 5, 2023.
This extension applies to travel to Canada through connecting flights from other countries, for example, if your flight itinerary starts in China and includes a connecting flight from Germany to Canada.
And those transiting through Canada with onward travel to another country.
If Canada isn't your final destination, also check the travel and testing requirements for your final destination.
However, this doesn't apply to travelers transiting through China, Hong Kong, or Macao, who are there for 24 hours or less.
For example, if your flight itinerary starts in Australia and includes a connecting flight in China before continuing to Canada, and if you are in China for 24 hours or less before the connecting flight to Canada is initially scheduled to depart.
And travelers must keep the proof of their test result until leaving the airport in Canada.
In the U.S., the Centers for Disease Control and Prevention issued an Alert - Level 2, Practice Enhanced Precautions, with similar requirements, on January 5, 2023.

The U.S. Food and Drug Administration (FDA) issued an updated Emergency Use Authorization (EUA) stating that the oral antiviral Paxlovid™ is now offered to people with a SARS-CoV-2 coronavirus infection without a confirmatory test.
On February 1, 2023, Patrizia Cavazzoni, M.D. Director Center for Drug Evaluation and Research with the FDA published EAU #105, confirming certain adults and pediatric patients can be prescribed Paxlovid without completing a diagnostic test.
However, there are known drug interactions that should be reviewed.
Pfizer previously confirmed Paxlovid is contraindicated in patients with a history of clinically significant hypersensitivity reactions to its active ingredients (nirmatrelvir or ritonavir) or any other components of the product and with drugs that are highly dependent on CYP3A for clearance and for which elevated concentrations are associated with serious and/or life-threatening reactions.
Therefore, before prescribing Paxlovid, clinicians should carefully review the patient's concomitant medications, including over-the-counter medicines, herbal supplements, and recreational drugs, says the U.S. NIH.
Pfizer Inc., Paxlovid's producer, stated on January 31, 2023, it expects a 58% reduction in related revenues in 2023.

The end of the Mosaico HIV vaccine trial in January 2023 must lead to a continued drive to innovate and an urgency to ensure that proven HIV prevention and treatment options reach all who need them stated UNAIDS.
Although no safety concerns were flagged during the HPX3002/HVTN706 clinical trial, it was discontinued after an independent review found no evidence of reduced risk of HIV infection among vaccinated participants.
The study evaluated an investigational vaccine regimen containing a mosaic-based adenovirus serotype 26 vector (Ad26.Mos4.HIV) administered during four vaccination visits over one year. In addition, a mix of soluble proteins (Clade C/Mosaic gp140, adjuvanted with aluminum phosphate) was also administered at visits three and four.
“The disappointment of the vaccine trial further underlines the importance of rolling out available HIV treatment and prevention innovations,” said UNAIDS Executive Director Winnie Byanyima in a press release on January 23, 2023.
“The search for a vaccine must continue, but it’s important to remember that despite this setback, the world can still end AIDS by 2030 by delivering all the proven prevention and treatment options to all the people who need them.
Rapid progress against the HIV pandemic is possible if existing prevention and treatment options are made available by sharing technologies, expanding provision, and tackling barriers to access.
The Joint United Nations Programme on HIV/AIDS (UNAIDS) leads and inspires the world to achieve its shared vision of zero new HIV infections, zero discrimination, and zero AIDS-related deaths.
The U.S. White House National Mpox Response recently noted that approximately 40% of people diagnosed with mpox also had HIV. In 2022, about one million people were vaccinated against Mpox in the USA.
As of February 3, 2023, HIV vaccine candidates continue clinical research studies.

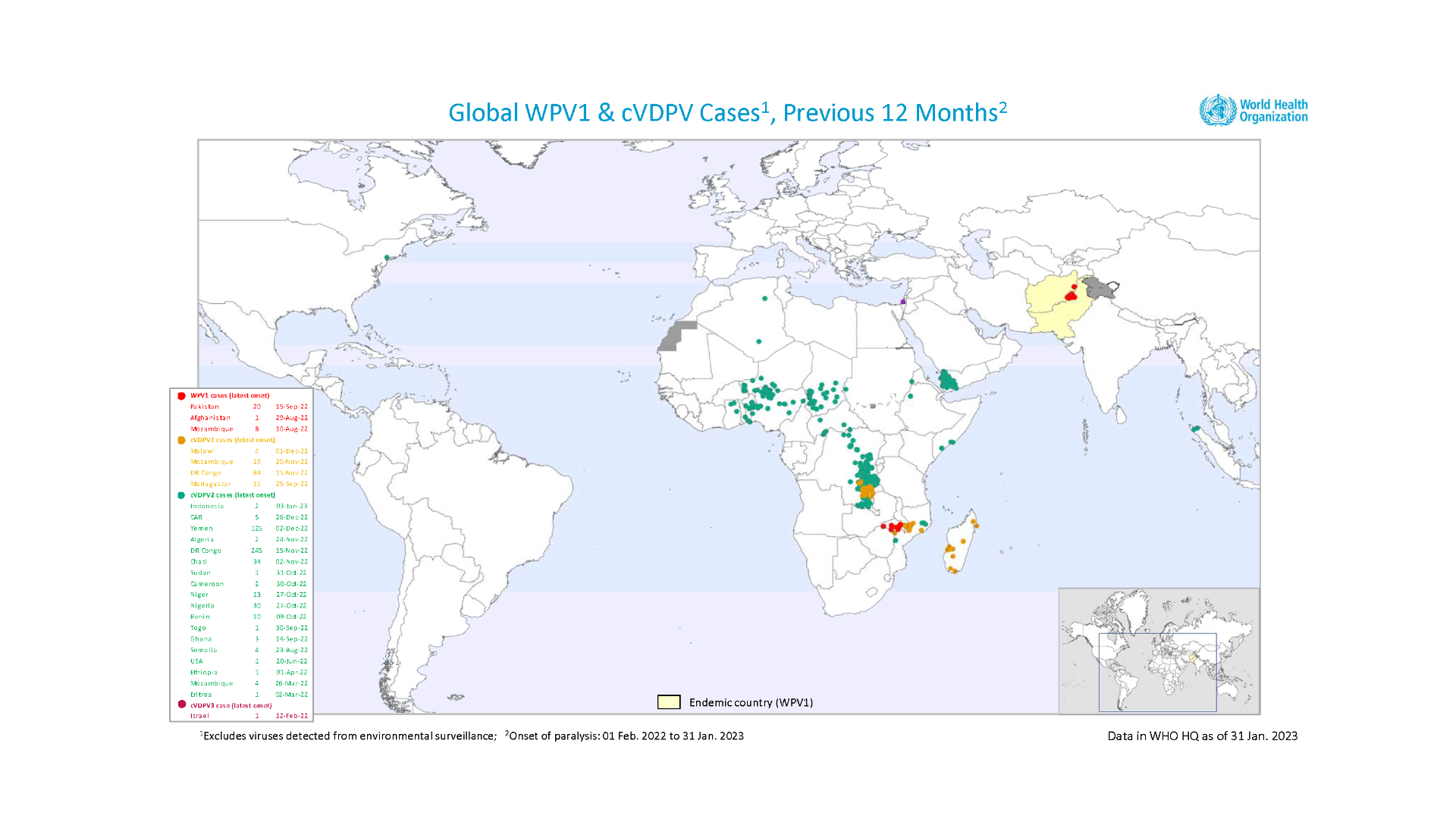
The World Health Organization (WHO) recently reaffirmed the spreading of poliovirus, and recent polio cases remain a public health emergency of international concern (PHEIC).
The WHO's Emergency Committee reviewed the data on wild poliovirus (WPV1) and circulating vaccine-derived polioviruses (cVDPV) in the context of the global target of eradicating WPV and cessation of outbreaks of cVDPV2 by the end of 2023.
Technical updates were received about the situation in the following countries: Afghanistan, Botswana, Canada, the Democratic Republic of the Congo, Indonesia, Madagascar, Nigeria, Pakistan, Sudan, and Zambia.
Although encouraged by the reported progress, the Committee unanimously agreed that the risk of the international spread of poliovirus remains a PHEIC and recommended the extension of Temporary Recommendations for a further three months into mid-2023.
The WHO Director-General endorsed the Committee's recommendations on February 1, 2023.
In the U.S., the Centers for Disease Control and Prevention (CDC) is leading the wastewater review for the continued spreading of poliovirus in New York, Michigan, and Pennsylvania in 2023.
The CDC stated in a Level 2 Travel Advisory posted on January 3, 2023, before traveling to any polio-risk destination, adults who previously completed the entire routine polio vaccine series receive a single, lifetime booster dose of polio vaccine.
Polio is a vaccine-preventable disease, says the CDC.
As a result, most clinics and pharmacies in the U.S. offer polio vaccination services in 2023.
According to reporting by Fierce Biotech, Merck Inc. confirmed on February 2, 2023, that it had discontinued the chikungunya vaccine candidate development program as part of a "routine pipeline prioritization."
While the vaccine candidate completed a phase 2 clinical trial, the study was suspended "due to a clinical stock recovery action," meaning it did not reach its original participant enrollment goal.
Merck's contender V184 was acquired through a $366 million takeover of Themis Inc. in 2020.
The chikungunya vaccine development race is led by Valneva SE, which completed the rolling submission of the VLA1553 vaccine's Biologics License Application to the U.S. Food and Drug Administration (FDA) in December 2022.
As of February 3, 2023, the FDA has not approved any chikungunya vaccine candidates.
This mosquito-transmitted disease remains a public health risk.
The Pan American Health Organization reported that in 2022, 87 fatalities were associated with chikungunya infections in the Americas last year.
Other chikungunya vaccine candidate news is posted at Vax-Before-Travel.com.

A leading online pharmaceutical discount company recently responded to a first-of-its-kind disciplinary civil action by the U.S. Federal Trade Commission (FTC).
The FTC alleged GoodRx was unauthorized when it shared health information with Meta Platforms Inc.'s Facebook and Alphabet Inc.'s Google, as well as other digital firms.
The FTC issued a $1.5 million fine as an enforcement action.
The FTC issued a policy statement in September 2021 warning health apps and others that collect or use consumers' health information that they must comply with the Health Breach Notification Rule.
"Digital health companies and mobile apps should not cash in on consumers' extremely sensitive and personally identifiable health information," said Samuel Levine, Director of the FTC's Bureau of Consumer Protection, in a press release.
"The FTC is serving notice that it will use all of its legal authority to protect American consumers' sensitive data from misuse and illegal exploitation."
On February 1, 2023, GoodRx published the following response:
'GoodRx, protecting our users' privacy is one of our most important priorities. Therefore, we are thoughtful and disciplined about what information we gather and how and why we use it.
The settlement with the FTC focuses on an old issue that was proactively addressed almost three years ago before the FTC inquiry began.
We do not agree with the FTC's allegations and admit no wrongdoing. However, entering into the settlement allows us to avoid the time and expense of protracted litigation.
We believe the requirements detailed in the settlement will have no material impact on our business or our current or future operations.
In fact, almost three years ago, before the FTC reached out to us, we proactively made updates consistent with our commitment to being at the forefront of safeguarding users' privacy.
While we used vendor technologies to advertise in a way that we believe was compliant with all applicable regulations and that remains a common practice among many health, consumer, and government websites, we are proud that we took action to be an industry leader in privacy practices.
We are glad to put this matter behind us so we can continue focusing on being a trusted source for Americans to find affordable and convenient healthcare.
The complete unedited response is available at this link.
More information on compliance and reporting breaches under the Health Breach Notification Rule are available on the FTC's Health Privacy page.
Disclosures: Precision Vax LLC participated in GoodRx programs until 2019 when it discontinued all business relationships.

A biotechnology company today announced positive results from a limited clinical trial evaluating a DNA vaccine candidate as a booster targeting the Zaire Ebolavirus.
This placebo-controlled Phase, 1b trial assesses its safety, tolerability, and immunogenicity in healthy adult participants who previously received a single injection of Merck's Ervebo®, a vaccine approved by the U.S. Food and Drug Administration for the prevention of disease caused by Zaire ebolavirus.
In the trial, INOVIO Pharmaceuticals, Inc.'s INO-4201 vaccine candidate was well-tolerated and boosted humoral responses in 100% (36 of 36) of study participants.
INO-4201 is a DNA vaccine targeting Zaire Ebola virus (ZEBOV) glycoprotein, designed to prevent ZEBOV infection. It encodes for a synthetic consensus antigen encompassing ZEBOV genetic variability from various outbreak strains to broaden immune coverage for divergent ZEBOV virus variants.
The participants were dosed with 1 mg of INO-4201 injected intradermally, followed by electroporation using our investigational proprietary smart device, CELLECTRA®.
Dr. Angela Huttner, MD, Infectious Disease Consultant, Geneva University Hospitals, and the study's lead investigator, commented in a press release on February 2, 2023, "INO-4201 was well-tolerated and all treated participants responded to the booster vaccine."
"These are encouraging results since our participants were initially vaccinated with Ervebo three to seven years ago."
"We remain grateful to our participants for their critical role in developing this vaccine candidate, which we hope will be a key player in future Ebola Virus Disease prevention."
This news is essential since recent research suggests dormant Ebola virus in a previously infected survivor could re-emerge up to nearly five years later and again allow human-to-human transmission Keita et al. Nature (Sept. 15, 2021).
The Ebola virus is classified as a Category A Priority Pathogen by the U.S. Centers for Disease Control and Prevention (CDC). This designation indicates a national security risk.
The Ebola virus family includes four virus species that cause periodic outbreaks of a highly contagious and lethal human infectious disease – called Ebola Virus Disease (EVD).
The virus is transmitted from wild animals to people and then easily spreads via human-to-human transmission.
Ebola outbreak news from 2022 and 2023 are posted at Vax-Before-Travel.com/Ebola.
The trial was spearheaded by Global Urgent and Advanced Research and Development, sponsored by Geneva University Hospitals, and funded by the U.S. Defense Advanced Research Projects Agency.
INOVIO is a biotechnology company focused on developing and commercializing DNA medicines. For more information, visit www.inovio.com.

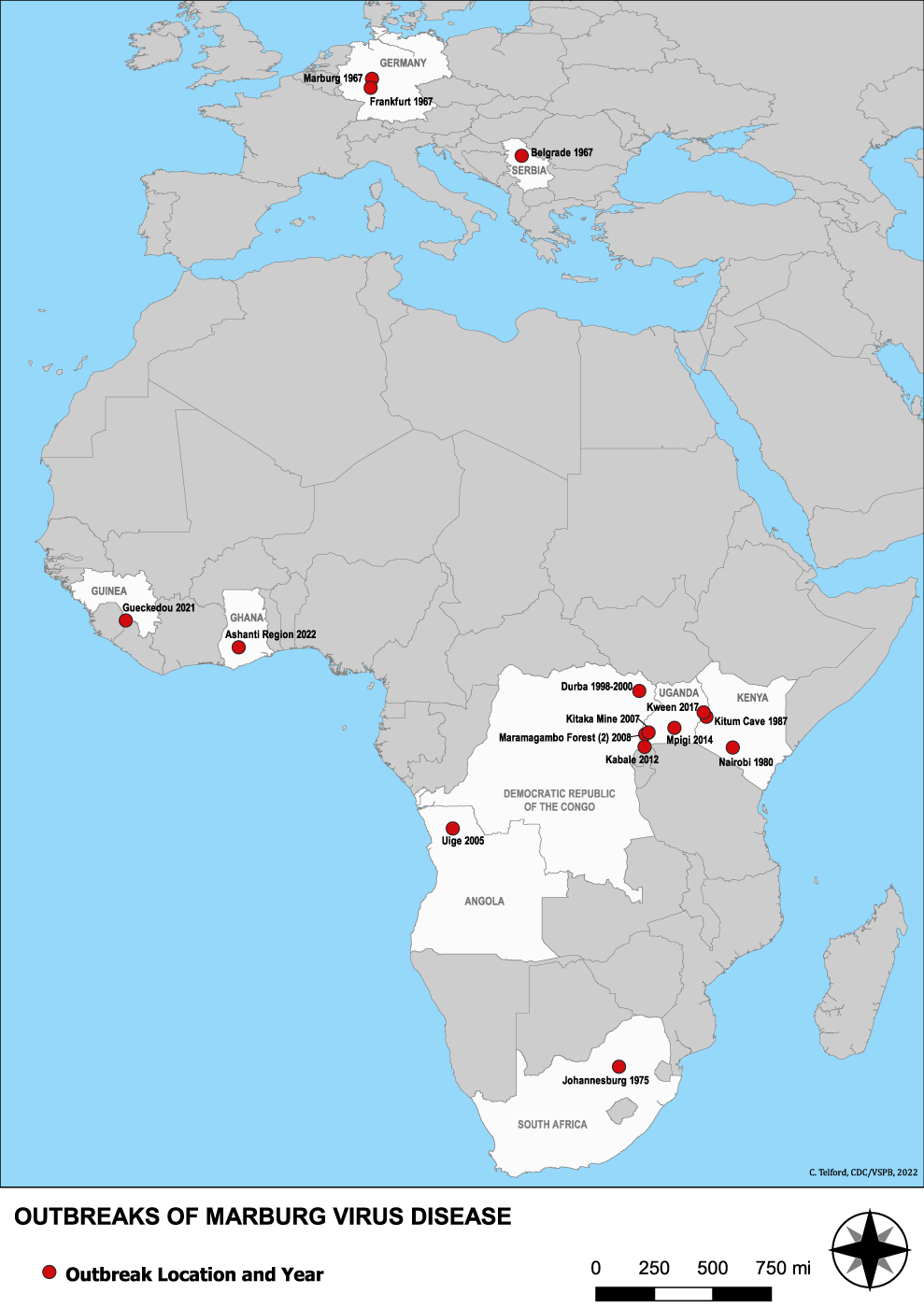
A Cincinnati-based biopharmaceutical company developing transformational vaccines today announced the development of vaccine candidates targeting Mpox and Marburg virus disease (“MVD”).
No vaccines or antiviral treatments are currently approved for MVD, which was first recognized in 1967.
As of 2023, Angola, DR Congo, Germany, Ghana, Guinea, Kenya, Serbia, South Africa, and Uganda have confirmed MVD cases.
Both candidate vaccines will utilize Blue Water Vaccines (BWV) Inc. norovirus shell and protrusion virus-like particle platform, which allows for the presentation of multiple antigens on the surface of either the S or P particle of a norovirus backbone.
In addition to monkeypox vaccine development, AbVacc will utilize its extensive expertise in MVD to develop a novel vaccine targeting the Marburg virus using BWV’s VLP platform.
“As various epidemics continue to emerge around the world, there has never been a better time to invest in the creation of preventative vaccines,” said Joseph Hernandez, Chairman and CEO of BWV, in a press release on February 1, 2023.
MVD is caused by either the Marburg or Ravn viruses, both from the same family as Ebola viruses and can cause outbreaks with high transmission and fatality rates.
According to the World Health Organization (“WHO”), Marburg spreads through human-to-human transmission via direct contact with the blood, secretions, organs, or other bodily fluids of infected individuals or contaminated surfaces.
Case fatality ratios of MVD can reach up to 88%, indicating a severe unmet need for preventative and therapeutic options, says the WHO.
Marburg vaccine candidates at listed on this webpage.