Search API
During the initial months of 2023, millions of people were frustrated by the lack of herpes simplex virus (HSV) vaccine development news.
As of February 28, 2023, the U.S. Food and Drug Administration has not authorized any HSV vaccine.
While herpes-candidate vaccines have been conducting clinical research for years, recent messenger RNA (mRNA) technology announcements raised hopes in 2022
These mRNA herpes vaccines are being produced by industry leaders Moderna Inc. and BioNTech SE.
Moderna's herpes simplex virus (HSV) vaccine candidate (mRNA-1608) is an mRNA vaccine targeted against HSV-2 disease, focused on inducing a strong antibody response with neutralizing functionality combined with cell-mediated immunity.
However, mRNA-1608 has yet to launch a human-based phase 1 clinical study.
Based on BioNTech's mRNA platform, the BNT163 candidate vaccine encodes three HSV-2 glycoproteins to help prevent HSV cellular entry and spread and counteract the immunosuppressive properties of HSVs.
BNT163's phase 1 clinical study includes 108 participants and was recently updated on January 5, 2023, and has an Estimated Study Completion Date of June 2025.
Herpes is a sexually transmitted disease (STD), says the FDA. There are two types of HSV that can cause genital herpes: HSV-1 and HSV-2.
Most cases of recurrent genital herpes are caused by HSV-2, and 11.9% of persons aged 14–49 years are estimated to be infected in the U.S.
However, an increasing proportion of anogenital herpetic infections have been attributed to HSV-1, which is prominent among young women.
To increase transparency, the U.S. National Instuties of Health and its partners launched STI Watch, a portal containing updated information on vaccine development status.
Other herpes vaccine news is posted at PrecisionVaccinations.com/Herpes.

Dynavax Technologies Corporation today announced that the United Kingdom's Medicines and Healthcare products Regulatory Agency had granted Marketing Authorization in Great Britain for HEPLISAV B® for the active immunization against hepatitis B virus infection (HBV) caused by all known subtypes of hepatitis B virus in adults.
"Hepatitis B is a highly infectious and potentially deadly virus with increasing infection rates, and over 250 million people infected worldwide. Thankfully, it can be prevented with effective vaccination," commented Ryan Spencer, Chief Executive Officer of Dynavax, in a press release on February 28, 2023.
HEPLISAV-B combines hepatitis B surface antigen with Dynavax's proprietary Toll-like Receptor 9 agonist adjuvant CpG 1018 to enhance the immune response.
HEPLISAV-B is indicated for preventing infection caused by all known subtypes of HBV in adults aged 18 years and older in the U.S.

Anixa Biosciences, Inc. today announced that the U.S. Patent and Trademark Office had issued a Notice of Allowance broadening the protection of Anixa's novel breast cancer vaccine technology.
This technology was invented by the late Dr. Vincent Tuohy and developed at Cleveland Clinic, and Anixa is the exclusive worldwide licensee.
Anixa's breast cancer vaccine candidate, currently in Phase 1 clinical trials, takes advantage of endogenously produced proteins that have a function at certain times in life but then become "retired" and disappear from the body.
One such protein is a breast-specific lactation protein, α-lactalbumin, which is no longer found post-lactation in normal, aging tissues but is present in most triple-negative breast cancers.
Activating the immune system against this "retired" protein provides preemptive immune protection against emerging breast tumors that express α-lactalbumin.
The vaccine candidate also contains an adjuvant that activates an innate immune response, which allows the immune system to mount a response against emerging tumors to prevent them from growing.
Dr. Amit Kumar, Chairman and CEO of Anixa, commented in a press release on February 27, 2023, "This breast cancer vaccine has the potential to prevent Triple Negative Breast Cancer, the deadliest form of breast cancer, and perhaps other forms of breast cancer that express alpha-lactalbumin."
"In addition, with our partners at Cleveland Clinic, we .... plan to present data from the trial at the annual American Association for Cancer Research meeting in April (2023)."
One in eight women in the U.S. will be diagnosed with invasive breast cancer at some point in their lives. Approximately 10-15% of those diagnoses are Triple-Negative Breast Cancer.

The World Health Organization (WHO) recently published its recommended composition of influenza virus trivalent and quadrivalent vaccines for use in the 2023-2024 southern hemisphere influenza season.
The recommendation, published on February 24, 2023, is based on data generated by the WHO Global Influenza Surveillance and Response System.
This periodic replacement of viruses contained in influenza vaccines is necessary for the annual flu shot to be effective against the evolving nature of influenza viruses.
During the current flu season in the U.S., all influenza vaccines are quadrivalent composition.
The most effective way to prevent the disease is vaccination, says the WHO. Moreover, flu shots provide protection, even when circulating viruses do not exactly match the vaccine viruses.
A recent analysis published on February 22, 2023, and MMWR issued on February 24, 2023, indicate the 2022-2023 flu shot preliminary effectiveness was about 68% protective for pediatric patients and 43% against adult hospitalization.
Breaking flu shot news is posted at PrecisionVaccinations.

The U.S. Centers for Disease Control and Prevention (CDC) recently concluded that reducing the number of persons traveling while infected with the SARS-CoV-2 virus could reduce associated transmission in airports, aircraft, and destination communities.
This CDC analysis published on February 24, 203, found post-arrival SARS-CoV-2 test results were 52% less likely to be positive when the predeparture COVID-19 testing requirement was in effect.
This finding was factual even when controlling for other factors, such as incidence in the flight’s country of origin and pool size.
Based on observed, real-world traveler data, these findings support the value of predeparture testing as a tool for reducing SARS-CoV-2 transmission associated with travel and were consistent with estimates from previous modeling studies.
The CDC continues to recommend testing before and after international travel.

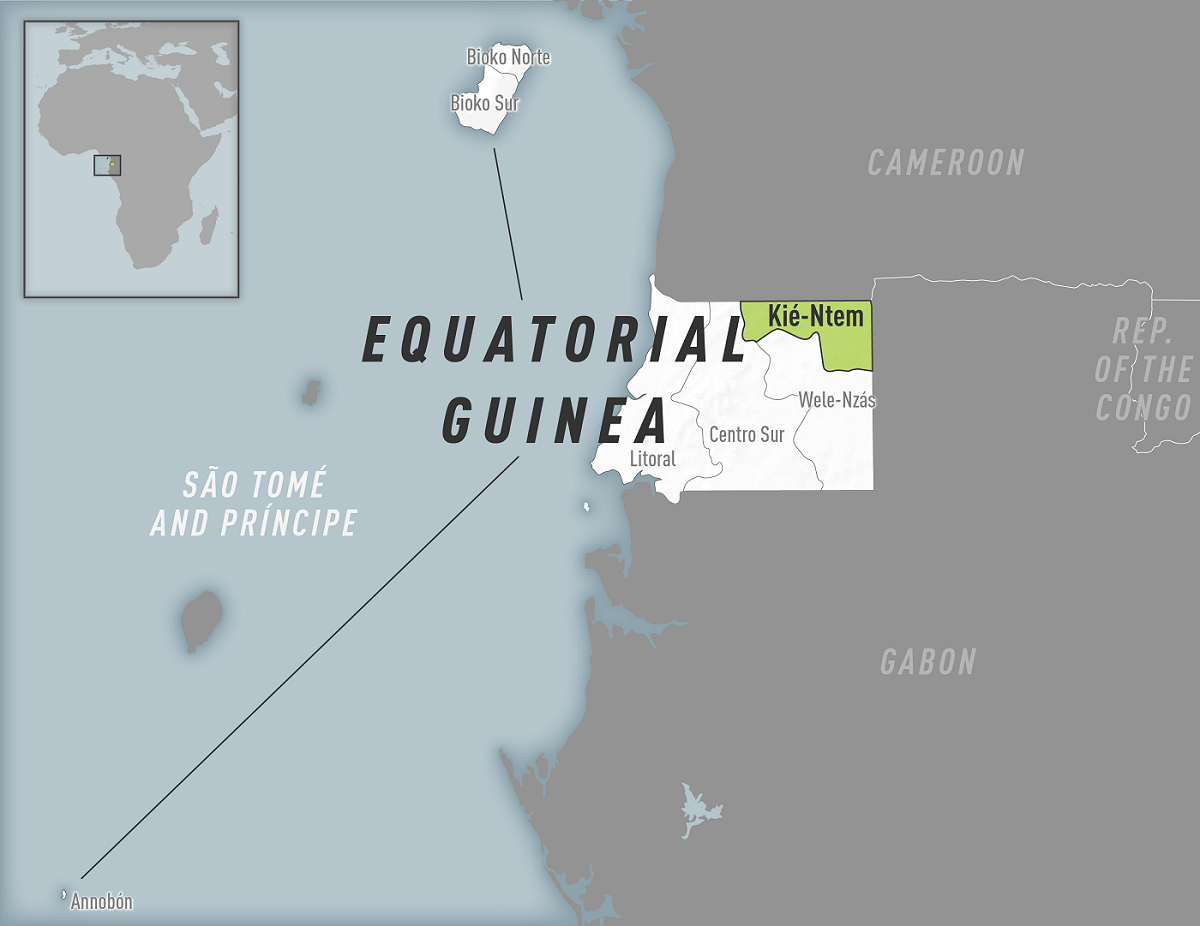
The World Health Organization (WHO) yesterday announced its investigations into Marburg virus disease (MVD) outbreaks are ongoing as additional cases have been reported.
This first MVD outbreak in 2023 was reported in Equatorial Guinea. As of February 21, 2023, the cumulative number of fatal MVD cases was nine.
Recently, Spain detected its first suspected MVD patient.
This man had recently visited Equatorial Guinea, reported VOA News on February 25, 2023.
Spanish health officials stated that more than 200 people in Equatorial Guinea have recently been quarantined because of MVD.
As of February 25, 2023, the WHO assesses the risk posed by MVD outbreaks as high at the national level, moderate at the regional level, and low at the global level.
Previously, the U.S. CDC issued a travel alert focused on these MVD outbreaks.
Marburg spreads through human-to-human transmission via direct contact (through broken skin or mucous membranes) with infected people's blood, secretions, organs, or other bodily fluids and surfaces and materials (e.g., bedding, clothing) contaminated with these fluids.
The incubation period varies from two to 21 days.
As of February 27, 2023, various MVD vaccine candidates are conducting clinical studies, but the U.S. FDA has not authorized any vaccine.
Update: Reuters reported Spain says patient does not have Marburg disease.
Moderna, Inc. and Merck recently announced that mRNA-4157/V940, an investigational personalized mRNA cancer vaccine, in combination with KEYTRUDA, has been granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration (FDA) for the adjuvant treatment of patients with high-risk melanoma following complete resection.
Personalized cancer vaccines are designed to prime the immune system so that a patient can generate a tailored antitumor response specific to their tumor mutation signature.
The FDA granted Breakthrough Therapy Designation based on positive data from the Phase 2b KEYNOTE-942/mRNA-4157-P201 trial.
"The FDA's Breakthrough Designation for mRNA-4157/V940 in combination with KEYTRUDA reflects the excitement that we have for the potential promise of individualized cancer treatments," said Stephen Hoge, M.D., Moderna's President, in a press release on February 22, 2023.
"mRNA-4157/V940 in combination with KEYTRUDA provided the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and potentially represents a new frontier in treating melanoma and other cancers."
"We look forward to publishing the full data set and sharing the results at an upcoming oncology medical conference, as well as continuing discussions with health authorities."
mRNA-4157/V940 is a novel investigational mRNA-based personalized cancer vaccine consisting of a single synthetic mRNA coding for up to 34 neoantigens that is designed and produced based on the unique mutational signature of the patient's tumor. Upon administration into the body, the algorithmically derived and mRNA-encoded neoantigen sequences are endogenously translated and undergo natural cellular antigen processing and presentation, a key step in adaptive immunity.
KEYTRUDA is an anti-programmed death receptor-1 (PD-1) therapy that works by increasing the ability of the body's immune system to help detect and fight tumor cells. KEYTRUDA is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes which may affect both tumor cells and healthy cells.
The FDA's Breakthrough Therapy Designation is granted to expedite the development and review of drugs that are intended to treat a serious condition, and when preliminary clinical evidence indicates that the product may demonstrate substantial improvement over available therapies on at least one clinically significant endpoint.
This news is essential since the rates of melanoma have been rising over the past few decades, with nearly 325,000 new cases diagnosed worldwide in 2020.
In the U.S., skin cancer is one of the most common types of cancer diagnosed, and melanoma accounts for a large majority of skin cancer deaths.
Xlear Inc. today announced it had supplemented the numerous scientific studies it has already provided the U.S. Department of Justice(DOJ) and the Federal Trade Commission (FTC) in the government's lawsuit against Xlear.
The DOJ-FTC sued Xlear alleging prior statements about nasal hygiene and COVID-19 lacked adequate substantiation.
As of February 27, 2023, Xlear stated in a press release it provided additional studies showing that simple nasal sprays are effective in helping reduce the transmission of (the SARS-CoV-2 virus) COVID-19 and reduce the severity of illness when used by individuals already infected with the virus.
One of the studies Xlear provided to DOJ is a peer-reviewed, double-blind, placebo-controlled, randomized clinical trial (RCT) involving 556 "high-risk healthcare professionals," finding that a nasal spray containing water, saline, and xylitol significantly reduced the transmission of COVID-19 and the severity of cases among those infected.
Specifically, the study finds: "[The spray] significantly reduced SARS-CoV-2 infection compared to placebo [36 cases (13.1%) Vs. 97 cases (34.5%); OR 0.29 (95% CI; 0.18–0.45), p < 0.0001].
Fewer clinical symptoms were also seen in the test group [57 cases (17.6%) vs. 112 cases (34.6%); OR 0.40, (95% CI; 0.27–0.59), p < 0.0001].
No harmful effects were associated with taking the [nasal spray] . . . This represents a 62% relative risk reduction in infection rate."
"[T]he spray was administered up to three times per day with . . . 6–8 [hour] between doses."
The study concludes: "[The nasal spray] has been shown to significantly reduce SARS-CoV-2 infections in healthcare workers, with 62% fewer infections when compared to placebo."
The company says, 'This product is not intended to diagnose, treat, cure, or prevent any disease.'


