Search API
The UK Health Security Agency Week #13 reported COVID-19 activity decreased across most indicators compared with the previous week.
As of March 30, 2023, through Respiratory Datamart, SARS-CoV-2 positivity decreased to 10% compared with 10.9% in the previous week.
Overall, COVID-19 hospitalizations decreased slightly in week #12 and were highest in the 85 and over age group.
Furthermore, the COVID-19 Autumn booster vaccination campaign commenced in September 2022.
By the end of week #10, about 65% of all people aged over 50 and living in England had been vaccinated with an Autumn booster dose.

The Region of the Americas has seen a significant surge in dengue cases and, in 2023, “is showing intense dengue transmission,” stated the World Health Organization on March 23, 2023.
By early March 2023, 342,243 dengue cases, including 86 deaths, were reported in the Americas.
At this pace, 2023 may easily eclipse the results seen in 2022.
In 2022, over 2.8 cases of dengue, including 1,290 deaths, representing a two-fold increase in patients compared with 2021.
Unfortunately, this trend is being realized in the state of Florida.
The Florida Health Department reported as of week #12, there had been 52 travel-associated dengue cases, and one locally acquired dengue case confirmed this year.
This compares with 903 travel-associated and 68 locally-acquired dengue cases reported in 2022, primarily in southern Florida.
The good news is that dengue is a vaccine-preventable disease, and two vaccines are becoming more available for international travelers in 2023.

Resistance to Paxlovid™ is already evident among viral SARS-CoV-2 variants currently circulating globally, indicating that this stand-alone antiviral, known as a protease inhibitor, could soon become less effective in treating COVID-19 patients.
Published on March 29, 2023, this study's conclusion was presented in the peer-reviewed journal Science Advances.
To lower the risk of resistance, the researchers say protease inhibitors must be carefully designed to avoid simple resistance mutations.
These researchers wrote that these results encourage the monitoring of resistance variants and the development of additional protease inhibitors and other antiviral drugs with different mechanisms of action and resistance profiles for combinatorial therapy.
"Despite Paxlovid's proven success in blunting COVID-19 symptoms, the long-term consequences of its widespread use in speeding up resistance are unknown," commented S. Arad Moghadasi, co-author of the study and a University of Minnesota Medical School graduate student in a press release.
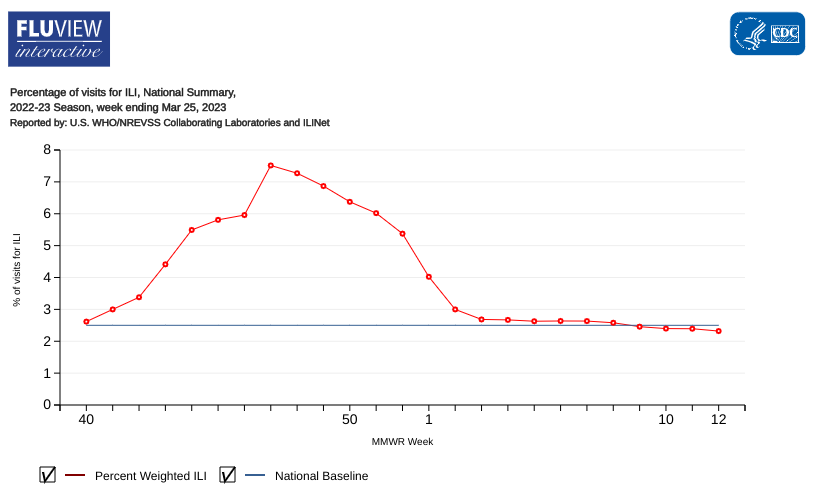
The U.S. Centers for Disease Control and Prevention (CDC) today announced four additional influenza-associated pediatric fatalities have occurred during the 2022-2023 flu season in the United States.
A total of 138 pediatric flu deaths have been reported so far this flu season.
The good news is the CDC FluView week #12 report confirmed seasonal flu rates were low nationally last week, with outpatient respiratory illness below baseline, and eight of 10 HHS regions are below their respective baselines as of March 31, 2023.
Previously, the World Health Organization published Influenza Update N° 401 on March 20, 2023, stating most indicators of influenza activity decreased to levels similar to or below levels typically observed this time of year in the Northern Hemisphere.
The CDC suggests anyone traveling to the Southern Hemisphere in April 2023 speak with a healthcare provider about potentially getting an additional flu shot for protection.
Flu shots remain available for most people over six months at most clinics and pharmacies in the U.S.
As of March 4, 2023, about 173.37 million influenza vaccines had been distributed during the 2022-2023 flu season.
The European Medicines Agency human medicines committee today announced it recommended authorizing the COVID-19 vaccine Bimervax (previously COVID-19 Vaccine HIPRA) as a booster in people aged 16 years and older who have previously been vaccinated with an mRNA COVID-19 vaccine.
It is the eighth vaccine recommended in the European Union for protecting against COVID-19 and, together with the vaccines already authorized, will support vaccination campaigns in EU Member States during the pandemic.
An updated listing of COVID-19 vaccines is posted at Coronavirus Today.

NSW Health today announced a Western Sydney infant infectious with measles spent time in locations in Parramatta and Westmead on March 27, 2023.
The infant, too young to be vaccinated against measles, acquired the infection in India before returning to Sydney.
NSW Health also confirmed a local measles case in September 2022.
Dr. Christine Selvey, Director of Communicable Diseases, NSW Health, commented in a press release on March 29, 2023, "Measles is a highly contagious infection, and the most vulnerable are infants under 12 months, who are too young to be vaccinated against it, other members of the community who are not fully vaccinated and people with a weakened immune system."
Measles cases increased worldwide by about 80% during 2022 compared with 2021.
Measles is a vaccine-preventable disease that quickly spreads in the air through coughing or sneezing by someone unwell with the disease.
"It can take up to 18 days for symptoms to appear after an exposure, so it is essential to stay vigilant if you've been in the above locations, and if you develop symptoms, please call ahead to your GP to ensure you do not spend time in the waiting room with other patients," Dr. Selvey added.
The U.S. Food and Drug Administration (FDA) recently approved the intramuscular route of administration for M-M-R®II and ProQuad®. In addition, the FDA approved the Priorix vaccine for use in the U.S.
Measles outbreak news for 2023 is posted at Vax-Before-Travel.

The start-up company PathoVax LLC announced that the U.S. Food and Drug Administration had concluded that a Phase 1 clinical trial for its monovalent component- HPV16 RG1-VLP vaccine RGVax may proceed.
This first-in-human, global multicenter Phase 1 clinical study seeks to demonstrate RGVax's safety and immunogenicity responses to HPV16 RG1-VLP in healthy volunteers.
RGVax is a chimeric HPV virus-like particle platform that displays 360 copies of the highly conserved, neutralizing HPV epitope (RG1).
The foundational technology is based on research conducted at the Johns Hopkins University and Medical University Vienna.
Unlike existing HPV vaccines, the RGVax technology and formulation have been shown to provide comprehensive protection against at least 18 high-risk human papillomavirus (HPV) types with immunogenicity lasting over a year without additional boosts in head-to-head studies with existing approved HPV vaccines.
"The world needs more, and particularly broad-based, HPV vaccines. We look forward to globally supporting these efforts in parallel and beyond this Phase 1, especially in Asia-Pacific and other developing countries, where there is a high burden of HPV diseases," said Dr. Kevin Koh, Chairman of PathoVax, in a press release on March 29, 2023.
A National Cancer Institute PREVENT contract funds the initiation of the Phase 1 study.
As of March 31, 2023, there are various approved HPV vaccines and several vaccine candidates in development.

The Janssen Pharmaceutical Companies of Johnson & Johnson recently disclosed that after assessing the respiratory syncytial virus (RSV) vaccine landscape, the Company would discontinue its RSV adult vaccine program.
“By periodically refocusing our portfolio, Janssen ensures that we are deeply invested in products that have the power to transform patients’ lives,” said Bill Hait, M.D., Ph.D., Executive Vice President, Chief External Innovation and Medical Officer and Interim Head, Janssen R&D., in a press release on March 29, 2023.
The decision to discontinue the RSV adult vaccine program is part of a broader effort to make strategic choices for its investments to focus on medicines with the greatest potential benefit to patients.
As of March 31, 2023, the U.S. FDA has not approved any RSV vaccine candidate.
However, the FDA previously approved monoclonal antibody therapies for infants.

The peer-reviewed journal Science Advances recently disclosed researchers from Tel Aviv University and the Israel Institute for Biological Research developed the first mRNA-based, lipid nanoparticle (LNP) vaccine effective against a lethal bacteria.
Published on March 8, 2023, this study on mice demonstrated that all vaccinated animals were fully protected against the bacteria that causes the plague.
Humans usually get the plague after being bitten by a rodent flea that is carrying the plague bacterium, says the U.S. CDC.
The most common sign of bubonic plague is the rapid development of a swollen and painful lymph gland called a bubo.
Plague morbidity and mortality rates have substantially decreased since the introduction of antimicrobials.
However, the isolation of Y. pestis strains resistant to multiple therapeutic antibiotics and the concern of a natural or intentional disease outbreak initiated by antibiotic-resistant strains emphasize the need to develop vaccines against the plague.
The researchers wrote, "Our mRNA-LNP vaccine elicited humoral and cellular immunological responses in C57BL/6 mice and conferred rapid, full protection against lethal Y. pestis infection after a single dose."
This study's findings suggest there is a new way of developing vaccines for bacterial diseases, including diseases caused by antibiotic-resistant bacteria.

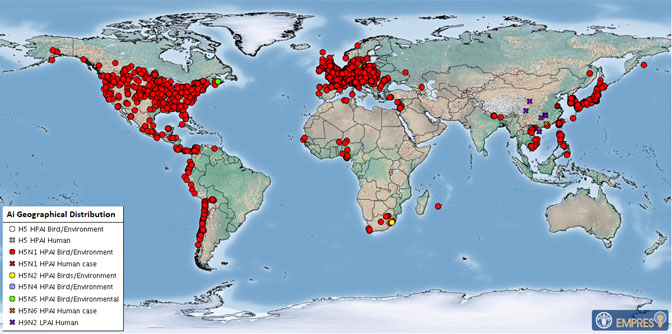
The Chilean Ministry of Health recently confirmed the country's first case of human infection with the avian influenza A(H5N1) virus.
This is the second human "H5N1 bird flu" virus infection reported in South America; Ecuador reported the first in December 2022.
Globally, this is the 11th human case of H5N1 reported since January 2022.
The prior 10 H5N1 cases also had exposure to poultry.
Bird Flu (Avian influenza) is a disease caused by influenza type A viruses that occur naturally among birds.
Detection of human infection with H5N1 bird flu in another country in South America is not surprising, wrote the U.S. Centers for Disease Control and Prevention (CDC) on March 29, 2023.
A recent CDC H5N1 technical report noted that "because of …the wide global prevalence of HPAI A(H5N1) viruses in wild birds and poultry outbreaks, continued sporadic human infections are anticipated."
As of early March 2023, H5N1 viruses (clade 2.3.4.4b) have been detected in mammals, wild birds, or poultry in 16 Latin America, the Caribbean, the United States, Canada, and most of the countries in the world.
In the U.S., about 6,300 people in 52 jurisdictions have been monitored since 2022, and only one human case has been identified in Colorado.
The CDC notes the annual flu shot does not offer protection from this type of influenza. However, there is an approved bird flu vaccine (Audenz™) should a human-to-human outbreak occur.