Search API
Given an increase in outbreaks caused by different microorganisms associated with medical and health tourism, the Pan American Health Organization / World Health Organization (PAHO/WHO) recently announced it encouraged Member States to strengthen their capacity to detect and manage related infections.
On July 7, 2023, the PAHO/WHO stated in recent years, the Region of the Americas has experienced an exponential growth of international travel in search of health care. For example, millions of Americans travel to other countries for health care each year, primarily to Mexico, Canada, and other countries in Central America, South America, and the Caribbean.
It is estimated that for the United States alone, the annual number has increased from 750,000 to 1.4 million over ten years (2007 to
2017).
Additionally, the U.S. Centers for Disease Control and Prevention (CDC), the Mexican Ministry of Health, and U.S. state health departments are responding to a multinational fungal meningitis outbreak among 35 people who had procedures in Matamoros, Tamaulipas, Mexico.
Ten U.S. patients have confirmed cases of fungal meningitis, and eight have died, according to the U.S. CDC.
The CDC says anyone who had procedures under epidural anesthesia from January to May 13, 2023, is at risk for fungal meningitis.... and should go to the nearest emergency room as soon as possible to be evaluated for fungal meningitis, even if you do not currently have symptoms.
Fungal meningitis is a rare, life-threatening fungal infection that causes swelling of the areas around the brain and spinal cord. Starting treatment right away if you are found to have fungal meningitis greatly increases the likelihood of survival.
However, infections are not contagious and are not spread from person to person, says the CDC.

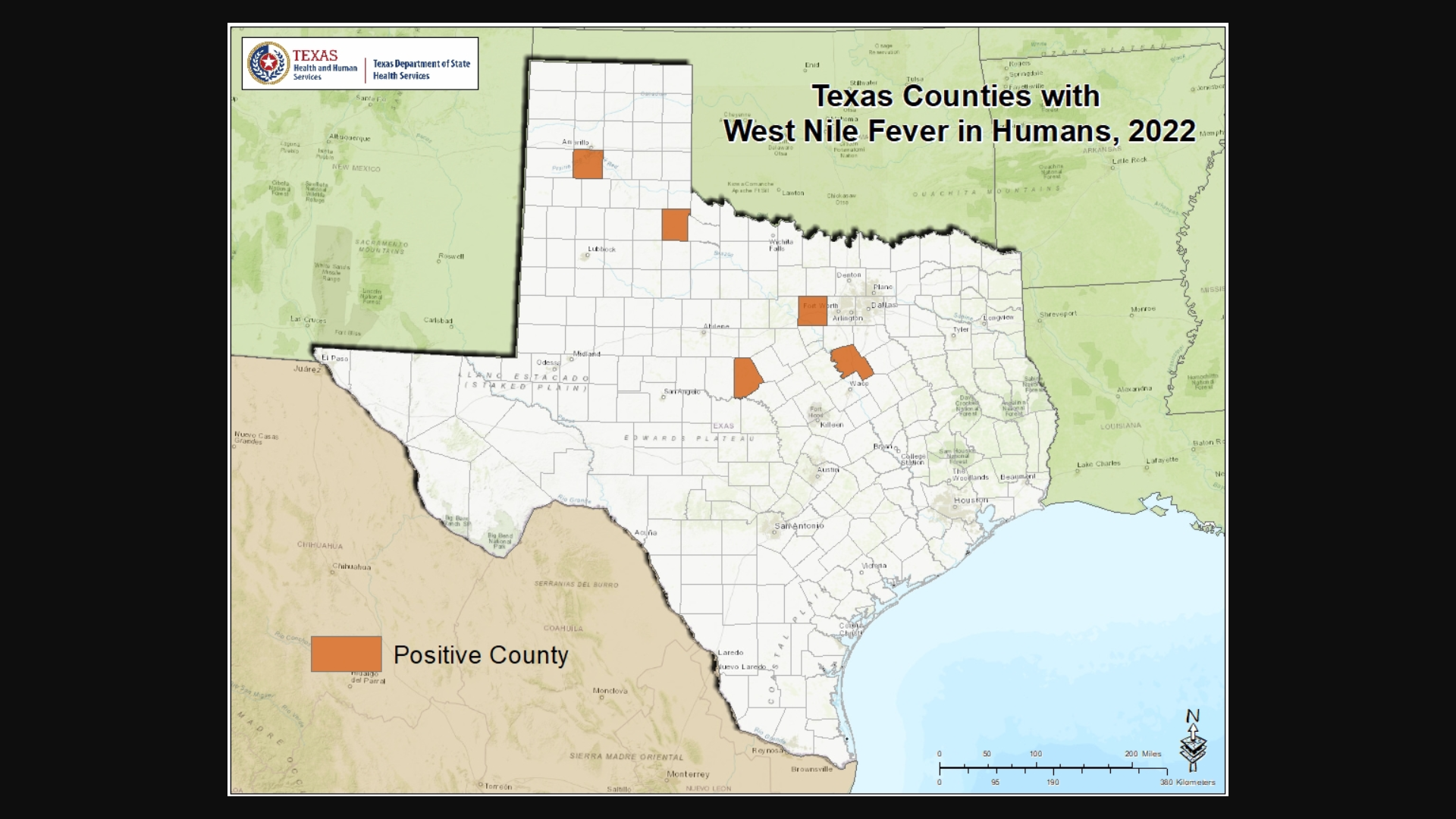
Dallas County Health and Human Services (DCHHS) today reported the first human case of West Nile Virus (WNV) in Dallas County for 2023.
The patient is a male resident and was diagnosed with West Nile Neuroinvasive Disease (WNND), which may affect the brain and spinal cord.
WNV is a disease that is spread by the bite of an infected mosquito. It is the leading cause of mosquito-borne disease in the U.S. The virus can infect humans, birds, mosquitoes, horses, and some other animals.
“WNV is transmitted to humans by the bite of an infected mosquito, and people should be careful when going out outside to enjoy outdoor activities,” said Dr. Philip Huang, DCHHS Director, in a press release on July 10, 2023.
Most people infected with WNV, about 80%, will not develop illness, says the Texas Health and Human Services.
Twenty percent of infected people develop a typically mild form of the disease known as West Nile fever, which may include fever, headache, body aches, and occasionally a skin rash on the trunk of the body and swollen lymph glands.
Only about one out of 150 people infected with West Nile virus will develop WNND.
As of late June 2023, there has been 10 WNND cases confirmed this year.
The U.S. CDC says there is no specific treatment for WNV infections nor is there an effective human vaccine.
The U.S. Centers for Disease Control and Prevention (CDC) recently issued a statement confirming when and explaining how the new COVID-19 vaccines will be available after September 2023.
The CDC stated on July 6, 2023, the new monovalent XBB.1.5 composition vaccines will be the first COVID-19 vaccines to be available directly from the pharmaceutical manufacturers as part of the commercial market rather than through the United States Government (USG).
It is anticipated that USG will stop the regular threshold/replenishment ordering mechanism for all COVID-19 vaccines and ancillary supplies on August 3, 2023.
Providers are encouraged to place necessary orders before that cutoff date.
Suppose a provider requires additional supply to be responsive to demand after USG closes the current ordering mechanism. In that case, COVID-19 vaccines will remain available for ordering via the established out-of-cycle request process.
The public will continue to be directed to Vaccines.gov to find providers offering COVID-19 vaccines.
CDC will provide access to COVID-19 vaccines for uninsured individuals once COVID-19 vaccines become commercially available.
Uninsured children can receive COVID-19 vaccines through the existing Vaccines for Children program.
And uninsured adults can receive COVID-19 vaccines through a new temporary program called the Bridge Access Program for COVID-19 Vaccines and Treatments.
As of July 7, 2023, more than 304.7 million doses of COVID-19 vaccine have been administered and reported by Federal Retail Pharmacy Program participants in the US. This includes 8 million doses administered onsite to long-term care facilities in the early days of the COVID-19 vaccination program.
Furthermore, detail on the current recommended vaccine schedules for each age group can be found on the CDC website.

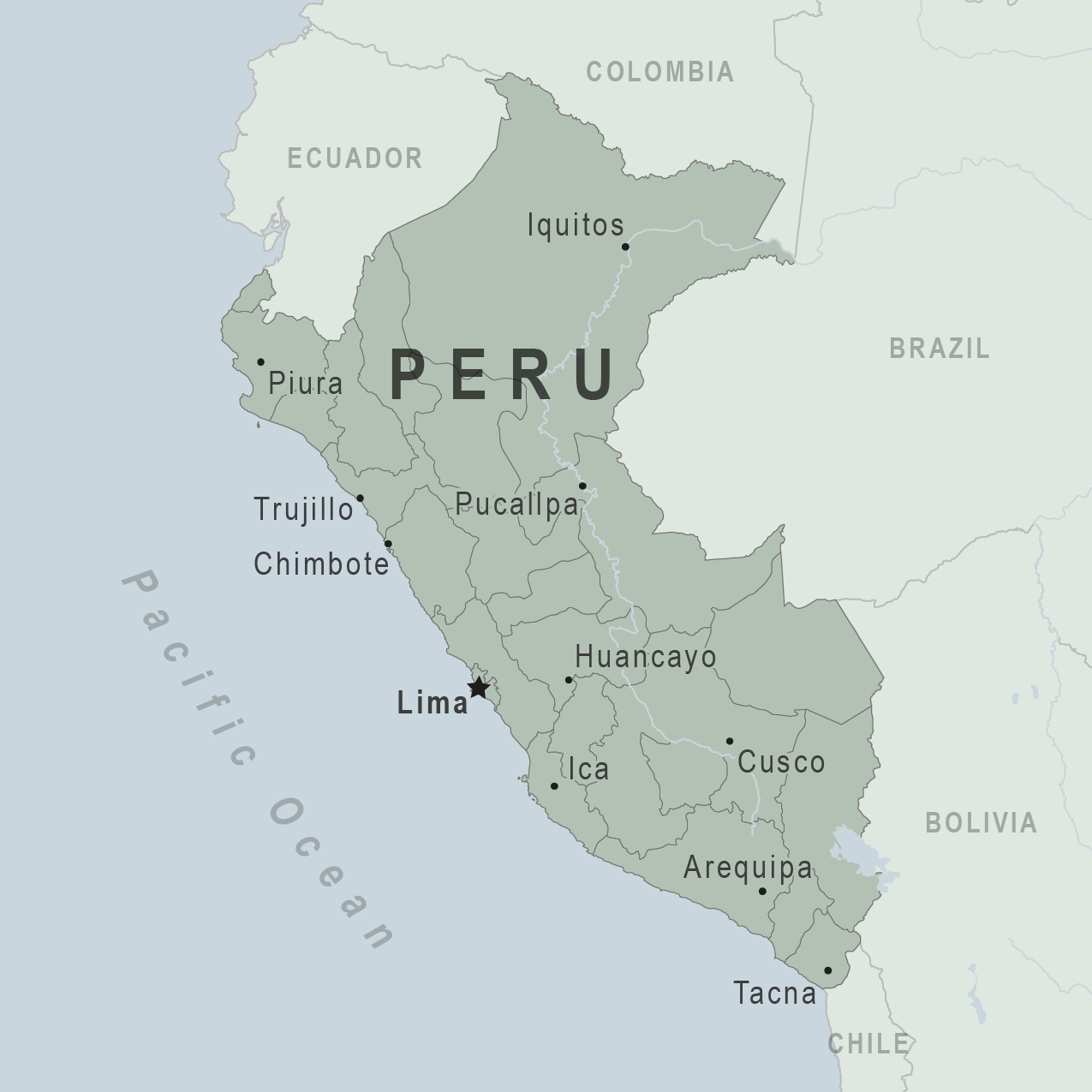
According to numerous reports, the Republic of Peru is confronted with another Guillain-Barré Syndrome (GBS) outbreak.
The Peruvian Government recently published Supreme Decree No. 019-2023-SA in the Official Gazette El Peruano, declaring a national health emergency due to the unusual increase in GBS cases in 18 of the country's 24 departments.
As reported on July 10, 2023, 182 cases of GBS have been confirmed, and four people have died since June 2023, per MercoPress.
Peru's president Dina Boluarte also issued a decree allocating $3.27 million for an action plan to improve patient care, including acquiring 5,000 immunoglobulin vials for treating patients affected by GBS.
Issued on July 8, 2023, this emergency declaration will be valid for 90 calendar days.
The U.S. Centers for Disease Control and Prevention (CDC) says GBS is the most common form of acute flaccid paralysis worldwide. It is characterized by motor weakness and other symptoms.
The CDC reported in a November 2020 Research Letter that from May 20–July 27, 2019, the Government identified 683 suspected or confirmed GBS cases in Peru.
Of the 683 GBS patients, 287 (42%) had descending muscle weakness, and 446 (65.3%) had ascending muscle weakness.
Of 426 patients for whom hospitalization data were available, 64 (15%) required mechanical ventilation.
Of 147 patients with an electrodiagnostic exam, 100 (68%) had acute motor axonal neuropathy.
The CDC stated this GBS outbreak was unusual because of the many cases. The incidence rate was nearly 25 times higher than expected.
And the rapid increase in numbers was followed by an equally precipitous decrease, which might suggest a point-source exposure.
Vaxart, Inc. recently announced positive topline data from the dose-ranging Phase 2 clinical trial of its oral pill bivalent norovirus vaccine candidate.
This study's preliminary results showed robust serum immune responses across all doses at Day 29 relative to Day 1.
Both doses showed a similar increase in serum antibody responses with no statistical difference between the medium and high dose arms, and the mucosal and cell-based assay data will be available later.
Dr. James F. Cummings, Vaxart's Chief Medical Officer, commented in a press release on July 6, 2023, "These data, additional forthcoming data from this study, and the data we expect from our norovirus challenge study, will help inform our selection of dosage levels in a larger Phase 2b study."
"And support an End-of-Phase 2 meeting with the U.S. Food and Drug Administration."
"Our bivalent vaccine is designed to target the most important genogroups, GI and GII, and specifically to cover the important strains, GI.1 and GII.4. GII.4 currently causes the majority of norovirus disease in humans."
This Phase 2 dose-ranging study demonstrated that the bivalent norovirus vaccine candidate was well tolerated, with a favorable safety profile.
This is the seventh clinical trial completed in Vaxart's norovirus program, and it supports previous findings of robust immunogenicity and benign tolerability.
As of July 10, 2023, Vaxart's vaccine is one of several norovirus vaccine candidates conducting clinical research.
Norovirus is a very contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus. The U.S. Centers for Disease Control and Prevention (CDC) says norovirus cases generally occur most frequently during late fall, winter, and early spring.
The CDC publishes the Norovirus Outbreak Map and posts Norovirus National Trends.

While official updates on H5N1-infected cats in Poland have increased over the past week, Polish authorities provided the European Centre for Disease Prevention and Control (ECDC) with an update, confirming that a total of 24 sick or dead cats were positive for influenza A(H5N1) virus (bird flu).
According to ECDC's testing guidance on avian influenza viruses in humans, any person exposed to sick/dead cats confirmed with A(H5N1) infection who develops symptoms should be tested as soon as possible for A(H5N1).
And persons exposed to sick/dead cats confirmed with A(H5N1) infection are advised to monitor their symptoms for 10–14 days after the last exposure and self-isolate if they develop symptoms.
They are also advised to wear a surgical mask or FFP2 respirator when in contact with others, seek medical advice and report it to public health authorities immediately.
And recently, the Italian Ministry of Health announced on July 6, 2023, that several dogs (and one cat) on a farm in Brescia, Italy, recently hit by avian influenza (bird flu), have seroconverted.
And the Italian Union of Public Medicine Veterinarians confirmed this HPAI H5N1 belonging to clade 2.3.4.4 b, and in particular to the H5N1-A/Herring_gull/France/22P015977/2022-like genotype, responsible for the cases reported in northern Italy in gulls.
This virus also has a mutation considered a marker of adaptation of mammalian viruses (T271A in the PB2 protein) with a possible increase in its zoonotic potential.
This mutation sparked considerable concern earlier this year when it was detected in infected mink in the fall of 2002, wrote the Avian Flu Diary.
The ECDC stated that considering the information and genomic data available until now and the fact that no human cases related to this event have been reported so far, ECDC assesses the current risk to the general public as low.
However, the risk is considered moderate for persons exposed to sick and/or dead cats confirmed with A(H5N1) infection, particularly if they belong to a vulnerable population group (immunocompromised people).
Considering the existing uncertainties, this assessment is preliminary and will be reviewed as soon as more information becomes available, says the ECDC.

The European Centre for Disease Prevention and Control recently published a Communicable Disease Threats Report (CDTR) for week #27, which included a mpox outbreak update.
The weekly number of mpox cases reported in the EU/EEA peaked in July 2022, and since then, a steadily declining trend has been observed.
Mpox is a viral disease, and the outbreak that began in May 2022 was driven by human-to-human transmission via close contact with infected individuals.
As of July 8, 2023, this CDTR confirmed since the last monthly update, 13 cases of mpox have been reported by Portugal (12) and Norway (1).
Portugal reported in the latest epidemiological update (June 30, 2023) that following three months with no new mpox cases, information is available for seven of the 12 patients; all were male, five (71%) were 20–29 years old, five presented with exanthema, and four are HIV-positive.
Based on evidence from the current outbreak and the declining number of new infections in the WHO European Region, the CDTR says the overall risk of mpox infection is moderate for men with sex with men and low for the broader population in the EU/EEA.
As of July 9, 2023, the leading mpox vaccine is JYNNEOS®.
As of June 27, 2023, 1,237,235 JYNNEOS doses (1st and 2nd) had been administered in 57 U.S. Jurisdictions. The U.S. CDC's vaccine advisory committee recently presented no recommendation for a third Jynneos dose.
Other sexually transmitted disease vaccine news is posted at Precision Vaccinations.

The Global Polio Eradication Initiative (GPEI) reported this week, three African nations reported continuing polio outbreaks.
Burkina Faso reported it's first circulating vaccine-derived poliovirus type 2 (cVDPV2) case of the year.
Chad reported two more cVDPV2 cases increasing its total for the year to ten.
And Nigeria reported six more polio cases, raising its total to 16 in 2023.
Furthermore, various countries reported cVDPV2-positive environmental samples as of July 5, 2023.
Previously, the World Health Organization (WHO) reconfirmed that the spread of poliovirus remained a Public Health Emergency of International Concern. As of July 2023, the WHO recommends travelers to polio-outbreak areas be fully vaccinated.
As of July 9, 2023, various polio vaccines are available worldwide.


