Search API
The U.S. Transportation Security Administration (TSA) recently disclosed its premium security service has slowed traveler screening.
TSA's new data indicated that in June 2023, 88% of TSA PreCheck® passengers waited less than 5 min.
Previously, TSA PreCheck was averaging over 92% of people passed through airport security quickly.
Furthermore, this performance decrease may not have been related to increased activity.
According to TSA's Passenger Volumes report for late June and July 2023, air travelers only equaled but did not exceed the pre-pandemic activity last seen in 2019.
TSA PreCheck® is currently available at more than 200 airports with 85+ participating airlines nationwide. 'We encourage you to check with your airline before each flight for the most up-to-date information', says the TSA.

The current outbreaks of avian influenza ("bird flu") continue to cause devastation in animal populations worldwide. Although largely affecting animals, these outbreaks pose ongoing risks to humans, says the World Health Organization (WHO).
The WHO wrote today that an increasing number of H5N1 avian influenza detections among mammals, which are biologically closer to humans than birds, raises concern that the virus might adapt to infect humans more easily.
In addition, some mammals may act as mixing vessels for influenza viruses, leading to the emergence of new viruses that could be more harmful to animals and humans.
Announced on July 12, 2023, the WHO, Food and Agriculture Organization of the United Nations, and the World Organisation for Animal Health urge countries to work together across sectors to save as many animals as possible and protect people.
Sporadic influenza A(H5N1) clade 2.3.4.4b virus detections in humans have been reported but remain very rare, with 8 cases reported since December 2021.
But only one case in the U.S.
Infections in humans can cause severe disease with a high mortality rate, says the WHO.
The human cases detected thus far are mostly linked to close contact with infected birds and contaminated environments.
"With the information available so far, the virus does not appear to be able to transmit from one person to another easily, but vigilance is needed to identify any evolution in the virus that can change that," said Dr. Sylvie Briand, Director of Epidemic and Pandemic Preparedness and Prevention, WHO, in a related press release.
"We encourage all countries to increase their ability to monitor these viruses and to detect any human cases. This is especially important as the virus now affects countries with limited prior experience in avian flu surveillance."
Studies are underway to identify any changes in the bird flu virus that may help the virus to spread more easily among mammals, including humans, says the WHO.
The U.S. CDC's Situation Summary issued as of July 5, 2023, confirmed the current risk to the public from bird flu viruses remains low. However, continued sporadic human infections are anticipated.
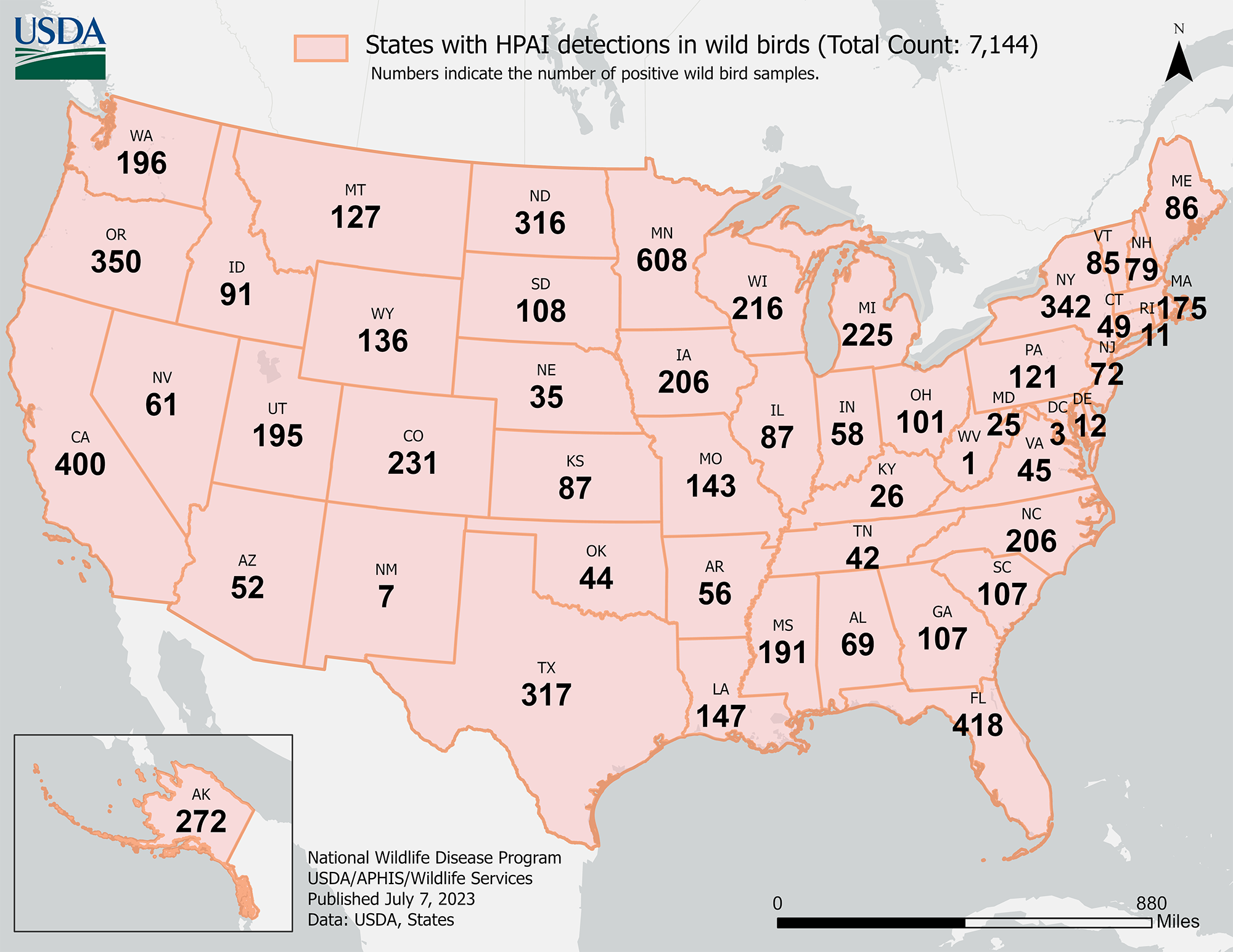
During this bird flu outbreak, there have been 7,105 virus detections in 47 U.S. states.
As of July 12, 2023, the U.S. government has stockpiled and approved avian influenza vaccines.
GSK plc recently announced that the Medicines and Healthcare products Regulatory Agency (MHRA) has authorized Arexvy for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in adults 60 years of age and older.
This is the first time an RSV vaccine for older adults has been authorized in Great Britain.
Neale Belson, GSK's Senior Vice President and General Manager UK, said in a press release on July 10, 2023, "Our ambition is to help protect adults 60 years of age and older in the U.K. who are at risk from RSV disease, including those with underlying medical conditions, who drive the majority of RSV hospitalizations."
"This authorization for Arexvy means eligible adults can be vaccinated against RSV disease for the first time, reinforcing GSK's long history of vaccine innovation."
RSV is a common, contagious respiratory virus that leads to an estimated 175,000 GP visits, 14,000 hospitalizations, and 8,000 deaths yearly in adults aged 60 and over in the U.K.
Recent studies indicate that the burden of RSV disease may be even greater than that of influenza in hospitalized older adults.
In the U.S., two RSV vaccines have been approved, and several RSV vaccine candidates are conducting late-stage clinical trials.
Precision Vaccinations publishes RSV seasonal trends for 2023.

The future vaccination plans for U.S. travelers and those living in dengue-outbreak areas, such as Puerto Rico, were disrupted yesterday.
Takeda announced on July 11, 2023, that the Company had voluntarily withdrawn the U.S. Biologics License Application (BLA) for its dengue vaccine candidate, TAK-003, following discussions with the U.S. Food and Drug Administration (FDA).
Takeda's press release stated that aspects of data collection could not be addressed within the current BLA review cycle.
On November 22, 2022, Takeda announced that the FDA had accepted and granted priority review of the TAK-003 BLA.
TAK-003, known internationally as QDENGA®, is approved in multiple endemic and non-endemic countries, such as the United Kingdom, Europe, and Brazil.
Gary Dubin, M.D., president of Takeda's Vaccines Business Unit, commented in a related press release, "The urgent global need to combat the growing burden of dengue remains, and we will continue to progress regulatory reviews and provide access for people living in and traveling to dengue-endemic areas while we work to determine next steps in the U.S."
QDENGA® is a tetravalent dengue vaccine preventing Dengue Fever or Severe Dengue caused by any of the four serotypes of the dengue virus.
During 2023, locally-acquired dengue has been confirmed in Florida and Texas.
While other second-generation dengue vaccine candidates are in development, the initial FDA-approved dengue vaccine Dengvaxia® remains available in the U.S. but has specific pre-vaccination requirements.

Dengue fever outbreaks continue to confront numerous countries in 2023. To extend access to dengue vaccines, the U.S. Food and Drug Administration (FDA) recently approved a supplement Biologics License Application (sBLA) for Dengue Tetravalent Vaccine, Live (Dengvaxia®).
On June 30, 2023, the FDA issued to Michael F. Stirr, Sanofi Pasteur, Inc. BL 125682/40, to include safety and efficacy data that support the use of Dengvaxia in individuals 6 through 16 years of age, with laboratory-confirmed previous dengue infection and living in endemic areas.
This FDA expansion is important as no specific medication to treat dengue infection exists.
As of July 3, 2023, the FDA's review of this supplement was associated with the following National Clinical Trial numbers: NCT01373281, NCT01374516, NCT00842530, and NCT01983553.
The three-dose Dengvaxia's (CYD-TDV) original BLA was approved by the FDA on May 1, 2019. It was first licensed in Mexico in 2015 for use in individuals 9-45 years of age and is now licensed in over 20 countries.
Dengvaxia is the only dengue vaccine recommended for routine use by the U.S. CDC's Advisory Committee on Immunization Practices.
Before being vaccinated with Dengvaxia, the CDC's vaccine committee says healthcare providers that if a patient has dengue symptoms or lives in or has recently traveled to an area with a risk of dengue and has not previously been infected, they are at increased risk for Severe Dengue disease when vaccinated and subsequently infected with the dengue virus.
Dengue is caused by infection with any of the four dengue viruses.
According to the CDC, these viruses are transmitted in tropical and subtropical regions by infected Aedes mosquito species.
During the summer of 2023, locally-acquired dengue cases have been reported in Florida, Texas, Puerto Rico, and Costa Rica.

Vaxcyte, Inc. today announced that the ongoing Phase 2 study of the VAX-24 pneumococcal conjugate vaccine (PCV) candidate in healthy infants is advancing to the second and final stage.
The Phase 2 study is evaluating the safety, tolerability, and immunogenicity of VAX-24, the Company’s lead, broad-spectrum 24-valent PCV designed to prevent invasive pneumococcal disease.
Vaxcyte’s Phase 2 infant study is being conducted in two stages and compares VAX-24 to the broadest-spectrum standard-of-care PCVs currently available.
Stage 1 of the study evaluated the safety and tolerability of a single injection of VAX-24 at three dose levels in a dose-escalation approach compared to VAXNEUVANCE™ (PCV15) in 48 infants.
The Stage 2 portion is evaluating the safety, tolerability, and immunogenicity of VAX-24 at the same three dose levels compared to PCV20 in approximately 750 infants.
In agreement with the U.S. Food and Drug Administration, Vaxcyte amended the study protocol for Stage 2 and changed the study comparator to PCV20, which is currently the broadest-spectrum PCV recommended by the U.S. CDC's Advisory Committee on Immunization Practices.
Grant Pickering, Chief Executive Officer and Co-Founder of Vaxcyte said in a press release on July 11, 2023, “We designed VAX-24 to deliver broader coverage and improved immune responses, and we look forward to sharing topline data from the primary three-dose immunization series by 2025", followed by topline data from the booster dose approximately nine months later.

Given an increase in outbreaks caused by different microorganisms associated with medical and health tourism, the Pan American Health Organization / World Health Organization (PAHO/WHO) recently announced it encouraged Member States to strengthen their capacity to detect and manage related infections.
On July 7, 2023, the PAHO/WHO stated in recent years, the Region of the Americas has experienced an exponential growth of international travel in search of health care. For example, millions of Americans travel to other countries for health care each year, primarily to Mexico, Canada, and other countries in Central America, South America, and the Caribbean.
It is estimated that for the United States alone, the annual number has increased from 750,000 to 1.4 million over ten years (2007 to
2017).
Additionally, the U.S. Centers for Disease Control and Prevention (CDC), the Mexican Ministry of Health, and U.S. state health departments are responding to a multinational fungal meningitis outbreak among 35 people who had procedures in Matamoros, Tamaulipas, Mexico.
Ten U.S. patients have confirmed cases of fungal meningitis, and eight have died, according to the U.S. CDC.
The CDC says anyone who had procedures under epidural anesthesia from January to May 13, 2023, is at risk for fungal meningitis.... and should go to the nearest emergency room as soon as possible to be evaluated for fungal meningitis, even if you do not currently have symptoms.
Fungal meningitis is a rare, life-threatening fungal infection that causes swelling of the areas around the brain and spinal cord. Starting treatment right away if you are found to have fungal meningitis greatly increases the likelihood of survival.
However, infections are not contagious and are not spread from person to person, says the CDC.

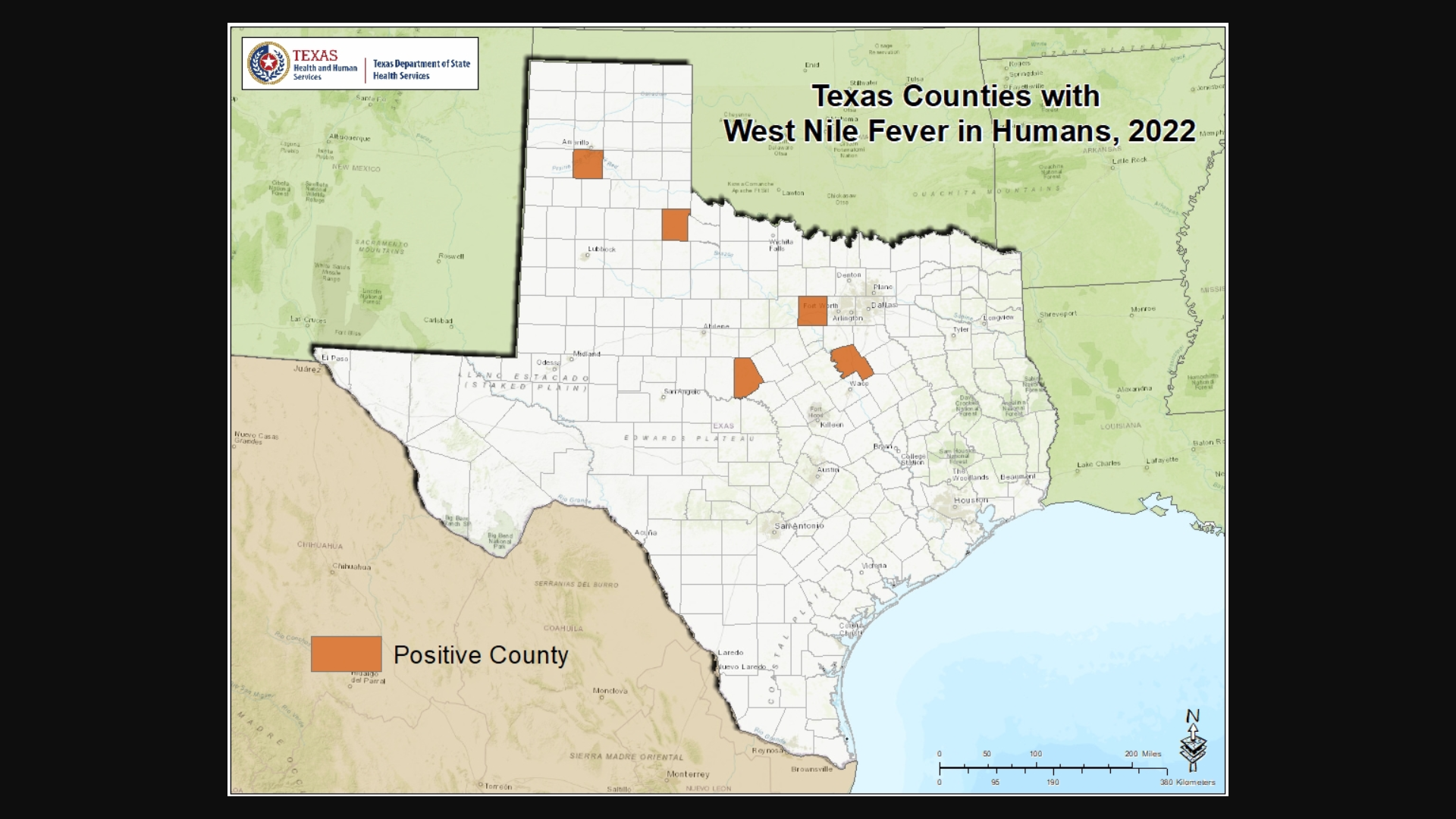
Dallas County Health and Human Services (DCHHS) today reported the first human case of West Nile Virus (WNV) in Dallas County for 2023.
The patient is a male resident and was diagnosed with West Nile Neuroinvasive Disease (WNND), which may affect the brain and spinal cord.
WNV is a disease that is spread by the bite of an infected mosquito. It is the leading cause of mosquito-borne disease in the U.S. The virus can infect humans, birds, mosquitoes, horses, and some other animals.
“WNV is transmitted to humans by the bite of an infected mosquito, and people should be careful when going out outside to enjoy outdoor activities,” said Dr. Philip Huang, DCHHS Director, in a press release on July 10, 2023.
Most people infected with WNV, about 80%, will not develop illness, says the Texas Health and Human Services.
Twenty percent of infected people develop a typically mild form of the disease known as West Nile fever, which may include fever, headache, body aches, and occasionally a skin rash on the trunk of the body and swollen lymph glands.
Only about one out of 150 people infected with West Nile virus will develop WNND.
As of late June 2023, there has been 10 WNND cases confirmed this year.
The U.S. CDC says there is no specific treatment for WNV infections nor is there an effective human vaccine.