Search API
A high-level travel advisory was reissued today for the Republic of the Union of Myanmar (Burma), disclosing various reasons not to visit this Southeast Asian nation in 2023.
On July 24, 2023, the U.S. Department of State's Level 4 Travel Advisory highlighted civil unrest and the risk of wrongful detention of U.S. nationals by the military regime exists.
Additionally, the U.S. government has limited ability to provide emergency services in Myanmar as U.S. government employees must obtain special authorization to travel outside of the city of Yangon (Rangoon).
Furthermore, visitors should exercise increased caution due to wrongful detentions and areas with land mines and unexploded ordnance.
From a health perspective, the State Department confirmed limited and/or inadequate healthcare resources in Myanmar.
And recently, the U.S. CDC confirmed Dengue is a risk in many parts of Asia and the Pacific Islands, including Myanmar.
If you intend to visit Myanmar, the CDC suggests speaking with a healthcare provider about routine and travel vaccinations.
Over the past eighteen months, numerous reports of avian influenza infections in birds, mammals, and even people in 47 states have been reported.
Since January 2022, based on its genetic features, about 836 flocks, and over 58 million birds have been infected with the highly pathogenic avian influenza (HAPI) virus.
To date, more than 6,500 people in 52 jurisdictions have been monitored since 2022, and only one human case has been identified in the U.S.
However, the U.S. Animal and Plant Health Inspection Service posted some excellent 'bird flu' news on July 25, 2023.
There has not been a confirmed bird flu outbreak in the U.S. since May 18, 2023.
On July 7, 2023, the U.S. CDC published an updated Technical Report that confirmed the overall risk to human health associated with the ongoing outbreaks of highly pathogenic A(H5N1) viruses in wild birds and poultry has not changed and remains low at this time.
Should a bird flu outbreak occur in humans, the U.S. government has already invested in developing related vaccines.
The World Health Organization (WHO) today published an update Disease Outbreak News regarding the Republic of Peru's ongoing Guillain-Barré Syndrome (GBS) outbreak.
On July 25, 2023, the WHO reported 130 suspected cases of GBS between June 10 and July 15, 2023. Of these cases (94 cases) presented with upward progression of paralysis as a neurological manifestation.
Throughout 2023 (until July 15, 2023), a total of 231 cases have been confirmed.
To date, the potential cause of the unexpected GBS incidence remains under investigation.
Furthermore, the WHO and the U.S. CDC have not issued any recommendations to impose travel and/or trade restrictions in response to this GBS outbreak.
The Presidency of the Republic of Peru declared a national health emergency in early July 2023, due to the unusual increase and enhanced the implementation of public health responses.
In 2019, Peru reported an unprecedented outbreak of GBS that affected several regions of the country, with almost 700 reported cases.
From the clinical-epidemiological characteristics and the study of the identified agents, it was concluded that the outbreak was associated with the presence of the Campylobacter jejuni sequence type 2993 genotype.
The WHO says GBS is a rare neurological disorder of variable clinical severity, including fatal outcomes. It is the most common form of acute flaccid paralysis (AFP), a polio-like disease.
It is characterized by motor weakness, areflexia (absence of muscle reflexes), sensory abnormalities, and elevated protein levels in cerebrospinal fluid (cytoalbuminologic dissociation). Most often, an upper respiratory or gastrointestinal illness typically precedes GBS.
No known cure for GBS nor protective vaccines is available, says the WHO.
Press Trust of India (PTI) today reported the Bacillus Calmette Guerin (BCG) vaccine manufactured by the Serum Institute of India will soon be exported to Canada for immunotherapy to treat bladder cancer.
BCG is a live freeze-dried preparation derived from an attenuated strain of Mycobacterium bovis.
As part of the therapy, the vaccine is administered into the bladder through a catheter, where it stays in the lining of the bladder for a specific duration affecting the cells and fighting cancer without impacting other body parts.
The product is for intravesical instillation and is available in 40 mg and 80 mg presentations, reported PTI PLB on July 25, 2023.
When cancer starts in the bladder, it is called bladder cancer, says the U.S. Centers for Disease Control and Prevention (CDC). Smoking is the most important risk factor for bladder cancer.
Since the 1970s, the BCG vaccines have been administered as an immunotherapeutic treatment for bladder cancer patients. And BCG has been the standard therapy for treating high-risk nonmuscle-invasive bladder cancer patients to avoid the recurrence and progression of the disease.
Using a catheter, BCG is given in a solution placed directly into the bladder. Intravesical treatments flush the bladder with drugs that kill cancer cells that remain after surgery.
According to the National Cancer Institute, this lowers the chance of cancer returning.
The 100 years old BCG vaccine is primarily used to prevent tuberculosis disease worldwide.
The journal NPJ Vaccines recently reported that researchers have developed and characterized a novel dual-target single-shot vaccine candidate that protects against Ebola (EBOV) and Yellow Fever (YFV) infection.
Announced on July 11, 2023, the YF-EBO pre-clinical vaccine candidate could help communities combat simultaneous EBOV and YFV epidemics, such as in Africa.
While there are approved vaccines for Ebola (Ervebo®) and Yellow fever (Stamaril®) in 2023, a single combo vaccine could enhance vaccination campaigns.
EBOV is a member of the Filoviridae family that causes severe and acute systemic disease in humans, known as Ebola virus disease, with mortality rates up to 80%.
YFV is a mosquito-borne flavivirus causing severe hemorrhagic disease in humans.
Yellow fever is endemic in Central and South America, as well as sub-Saharan Africa, where EVD surges.
Despite the availability of a very efficient live-attenuated yellow fever vaccine (Stamaril), annually, an estimated 51,000–380,000 severe cases of YF still occur, resulting in 19,000–180,000 deaths.
The re-emergence of YF outbreaks can be mainly attributed to low vaccine coverage due to supply issues.
Therefore, alike for EVD, a second-generation YFV vaccine with a sustainable supply could deliver measurable benefits during dual outbreaks.
Future studies must address whether this observed cross-reactive humoral immunity is sufficient to also provide cross-protection against heterologous challenge, ideally in step-up models using original filoviruses under BSL4 conditions, wrote these researchers.

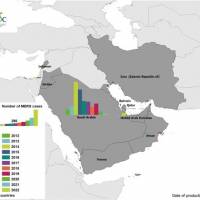
The World Health Organization (WHO) today announced a case of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) cases was confirmed in Abu Dhabi, the United Arab Emirates (UAE).
According to the WHO's Disease Outbreak News on July 24, 2023, this MERS-CoV case had no history of direct or indirect contact with camels, goats, or sheep.
Prior to this WHO notification, the last MERS-CoV infection reported from the UAE was in November 2021.
Since July 2012, the UAE has confirmed 94 MERS-CoV cases and 12 related fatalities.
The WHO expects that additional cases of MERS-CoV infection will be reported from the Middle East.
MERS-CoV cases have reached 2,605 globally, including 936 associated deaths as of July 2023.
These are MERS vaccine candidates in development, but the WHO has not approved any.

Bavarian Nordic A/S recently announced that its Phase 3 clinical trial of MVA-BN® RSV, a respiratory syncytial virus (RSV) vaccine candidate for adults ≥60 years of age, did not meet all the primary endpoints of preventing lower respiratory tract disease (LRTD) from RSV.
Based on this outcome, Bavarian Nordic will discontinue its RSV program, including its partnership with Nuance Pharma to develop and launch the vaccine for selected Asian markets.
“We are disappointed that our RSV vaccine candidate was not successful in this pivotal trial,” said Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, in a press release on July 22, 2023.
The final study results showed that the vaccine candidate had a 59% efficacy in preventing at least two pre-defined LRTD symptoms meeting one of the efficacy criteria of the study.
However, when measuring more severe LRTD based on at least three pre-defined symptoms, the vaccine candidate only demonstrated a 42.9% efficacy and missed the co-primary endpoint of the study.
As of July 24, 2023, there are two approved RSV vaccines in the U.S. and several late-stage vaccine candidates conducting research.

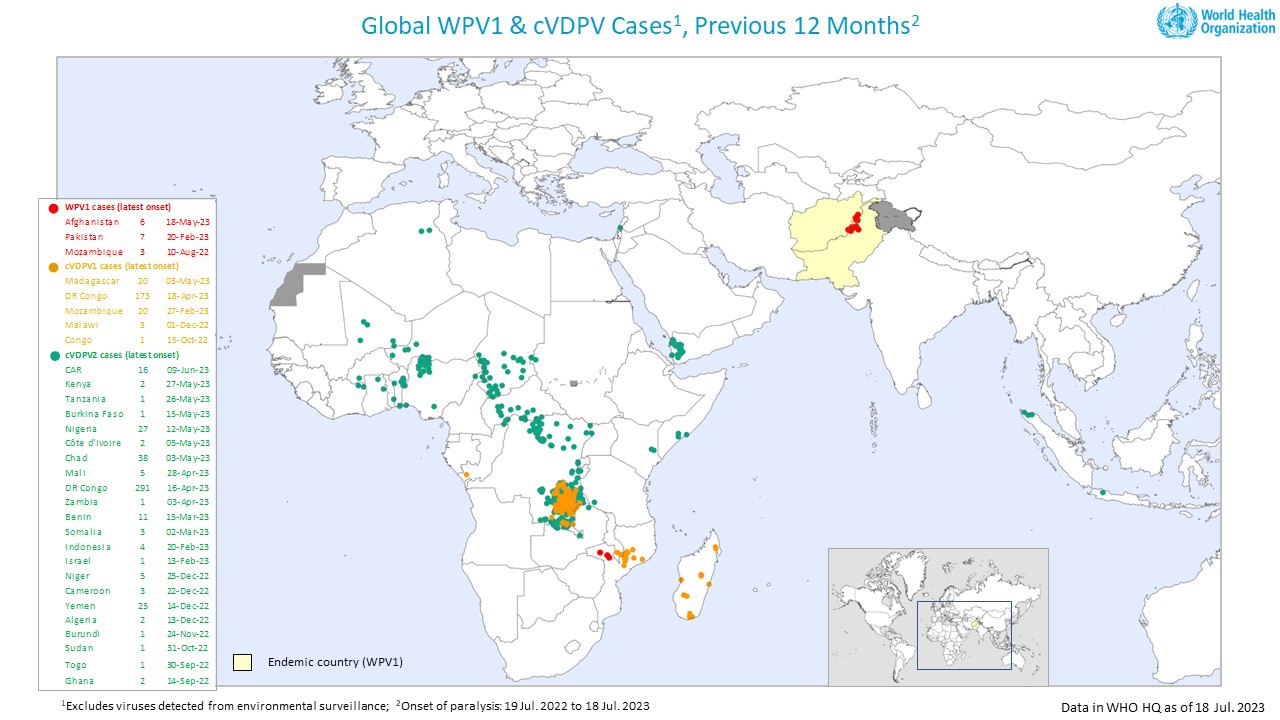
With 117 confirmed cases of circulating variant polioviruses and 107 detections in sampled wastewater in the WHO African Region as of July 2023, the Africa Regional Certification Commission (ARCC) recently urged partners to address gaps in polio immunity urgently.
The ARCC, which held its 31st meeting in early July 2023, called for accelerated implementation of supplementary immunization activities.
"We are looking forward to implementing the additional ARCC recommendations to guide how we can deliver on the promise of the polio-free Democratic Republic of the Congo and Africa," said Dr. Serge Emmanuel Holenn, Deputy Minister of Health of the Democratic Republic of the Congo.
In this response, @WHOAFRO Tweeted on July 24, 2023, that the Republic of Madagascar recently launched a polio vaccination campaign to reach over 18 million people.
Madagascar has reported 13 cVDPV1 cases in 2023. Last year, there were 16 cVDPV1 cases.
And 8 circulating vaccine-derived poliovirus type 1 (cVDPV1) positive environmental samples were reported in Analamanga in July 2023.
Countries with cVDPV1 have the risk of international spread. Therefore, they are subject to WHO temporary recommendations.
The World Health Organization (WHO) says polio is a vaccine-preventable disease.
As of July 2023, 670 million doses of the nOPV2 vaccine had been administered, primarily in Africa. On March 28, 2023, the WHO's SAGE recommended that it be the preferred polio vaccine for response to cVDPV2 outbreaks wherever possible.
The nOPV2 vaccine is not authorized by the Food and Drug Administration. Therefore, it is not available in the U.S.
The ARCC is an independent body established in 1998 to oversee the certification status of the African region as free from indigenous wild poliovirus.
Updated: On July 25, 2023, the WHO confirmed the priority regions (Analamanga, Vakinankaratra, Alaotra Mangoro, and Atsimo Andrefana) and those under 15 in the 19 other regions.