Search API
While the global flu season has not been very severe, recent data indicates the southern United States is experiencing a sudden spike in influenza cases.
According to the U.S. Centers for Disease Control and Prevention (CDC), during week #45, 3.5% of patient visits reported were due to respiratory illness that included fever plus a cough or sore throat.
As of November 17, 2023, the regions with the highest percentage of respiratory specimens testing positive for influenza were Regions 8 (7.4%), 4 (7.0%), 6 (6.5%) and 9 (5.3%).
For regional and state-level data and age group distribution, please visit FluView Interactive.
From a severity perspective, the National Center for Health Statistics Mortality Surveillance confirmed that during week #44, 21 people died from influenza.
Previously, influenza deaths were reported during weeks #43 (32) and #42 (43).
Furthermore, one influenza-associated pediatric death occurring during the 2023-2024 season has been reported to the CDC.
The CDC continues recommending most people over six months of age get an annual flu vaccine.
From an availability perspective, over 147 million flu vaccine doses have been distributed in the U.S. Various flu shots are available at clinics and pharmacies in the U.S.

The U.S. Centers for Disease Control and Prevention (CDC) today published a Morbidity and Mortality Weekly Report revealing that global coverage of the measles-containing vaccine (MCV) has declined to the lowest levels since 2008.
The CDC confirmed that from 2000 to 2022, measles vaccination prevented approximately 57 million deaths worldwide.
However, there has been an 18% increase in estimated measles cases and a 43% increase in estimated measles deaths in 2022 compared with 2021.
Measles is highly contagious, infecting about 90% of people following exposure to the virus, says the CDC.
As of November 17, 2023, disruptive measles outbreaks were reported globally.
All six World Health Organization regions have committed to eliminating measles; however, no region has achieved and sustained measles elimination.
The CDC recently reported that Yemen (28,247) and India (24,301) have reported the most measles cases over the past year.
Measles is a highly contagious, vaccine-preventable disease that requires high population immunity for transmission to be interrupted.
Measles vaccination programs began in the United States in 1963 and continue in 2023.
As of November 2, 2023, a total of 41 measles cases were reported by 18 jurisdictions.
The majority of these measles cases are related to international travelers.
Furthermore, the CDC published a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice in September 2023, identifying measles outbreaks in 39 countries.
On August 17, 2023, the CDC conducted a COCA webinar urging all healthcare providers to ensure their patients were up to date on the measles vaccination.
Various measles vaccines are available worldwide. In the U.S., most clinics and pharmacies offer measles vaccination services.

PharmaJet® today announced the start of the first human clinical trial for a Venezuelan Equine Encephalitis (VEE) vaccine delivered with PharmaJet Precision Delivery Systems.
The Phase 1 study, sponsored by PharmaJet, aims to identify the optimal dose, vaccination schedule, and delivery system most suitable for use in subsequent broader clinical evaluations of the VEE DNA vaccine candidate.
VEEV is a mosquito-borne alphavirus that has caused sporadic outbreaks and epidemics in North Central and South America. Aerosolized VEEV is highly infectious with greater mortality rates than natural infection and is listed as a potential biothreat agent with no approved human vaccine or therapeutic.
Disease outbreaks frequently involve equines– horses, donkeys, mules, zebras – and humans.
DNA vaccination has proven particularly effective at eliciting protective immune responses against the alphavirus challenge.
As previously reported, the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) and PharmaJet found, in a non-human primate challenge study, that a prime and single boost by either the intramuscular (Stratis) or intradermal route (Tropis) resulted in humoral and cellular immune responses that provided significant protection against VEEV disease and viremia.
These results paved the way for advancing the candidate vaccine into human trials and approval to proceed was recently granted by the Institutional Review Board (IRB), the Army's Office of Human Research Oversight, and the FDA.
President and CEO of PharmaJet, Chris Cappello, said in a press release, "The PharmaJet Precision Delivery Systems are optimized for field use and have also shown immunogenicity levels higher than with needle-syringe."
"We look forward to the clinical results from the human trial of this promising DNA vaccine candidate with our commercially available needle-free precision delivery systems."
The VEE DNA vaccine candidate is being evaluated as part of a multi-year agreement between PharmaJet and the Joint Science and Technology Office of the U.S. Defense Threat Reduction Agency.
The study, in collaboration with USAMRIID, aims to advance further the clinical assessment of the vaccine with the PharmaJet Precision Delivery Systems: Tropis Intradermal and Stratis Intramuscular.
PharmaJet Systems effectively delivers nucleic acid-based vaccines compatible with military operations and the warfighter environment, in addition to being preferred by end-users compared to other delivery technologies.

According to IDSE News, the recently approved half-life monoclonal antibody offering passive immunization to prevent lower respiratory tract infections in infants caused by the respiratory syncytial virus (RSV) has increased availability.
The U.S. Centers for Disease Control and Prevention (CDC) recommends passive immunization to protect infants under eight months and some older babies at increased risk of severe illness caused by RSV.
“CDC and FDA are committed to expanding access to this important immunization so that more parents have peace of mind during the winter virus season,” said Dr. Nirav D. Shah, CDC’s principal deputy director, in a press release.
On November 16, 2023, the CDC released more than 77,000 additional 100-mg doses of Beyfortus™ (Nirsevimab-alip) to physicians and hospitals through the Federal Vaccines for Children (VFC) Program and commercial channels.
The co-producer of Beyfortus, Sanofi, stated in October 2023 that demand for this RSV product had been higher than anticipated.
On October 23, 2023, the CDC published a Health Alert Network Health Advisory to provide options for clinicians in the context of a limited supply of Betforus. The CDC previously announced it had transitioned to an allocation-based system for distributing Beyfortus.
As of October 2023, Sanofi's price for Beyfortus through the VFC program is $395.00 for 100mg and $395.00 for 50mg through March 2024.
AstraZeneca CEO Pascal Soriot recently informed Reuters, "We've had to deliver what is needed (for the U.S.), and next year, the volume suddenly will go up quite a bit."
Globally, the 2023 RSV season's activity was generally low or decreasing except in some Europe, Central America, Caribbean countries, and the United States as of mid-November 2023.
In the U.S., there are variations in the timing of RSV outbreaks between regions and between communities in the same area, says the CDC.
Note: news article updated on Nov. 18, 2023, to include CDC quote.

Longhorn Vaccines and Diagnostics today presented new data from an animal study of LHNVD-301, the company's lead tuberculosis (TB) vaccine candidate.
The data presented at The Union World Conference on Lung Health 2023 on November 15, 2023, showed that a mycobacterium tuberculosis (MTB) vaccine could generate broader protection against pathogens susceptible to antimicrobial resistance (AMR).
LHNVD-301 is an unconjugated, peptide-based vaccine that combines a MTB heat shock protein epitope and a peptidoglycan (PGN) epitope.
PGN is a cell wall component of bacteria that plays a vital role in infections.
The combination of heat shock protein and PGN generates broad reactive antibodies.
It represents a novel approach that combats AMR while targeting tuberculosis by combining multiple epitopes specific to MTB and common to gram-positive bacteria into a peptide vaccine.
Longhorn CEO Gerald W. Fischer, MD, commented in a press release, "We are developing LHNVD-301 to address MTB as a global threat and combat AMR."
"Unconjugated peptides targeting epitopes of multiple pathogens enable a cost-effective, easily scalable approach for vaccine development, which is crucial for covering those most at risk for TB."
While TB is an ancient disease, outbreaks caused an estimated 10.6 million infections and 1.3 million deaths in 192 countries and areas in 2022.
As of November 2023, 16 TB vaccines are available globally, and various BCG vaccine candidates are under development.

UCB announced today that BIMZELX® is commercially available for treating moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
BIMZELX is the first and only approved psoriasis treatment (humanized IgG1 monoclonal antibody) designed to selectively inhibit two key cytokines driving inflammatory processes – interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
BIMZELX (bimekizumab-bkzx) is available as an autoinjector and a pre-filled syringe.1 BIMZELX may be administered subcutaneously by a healthcare professional, or a patient may self-inject after proper training.
The U.S. Food and Drug Administration approved BIMZELX on October 17, 2023.
People living with moderate-to-severe plaque psoriasis should talk to their healthcare provider to see if BIMZELX may be right for them. However, avoid the use of live vaccines in patients treated with BIMZELX.
"While the treatment landscape for psoriasis has evolved in recent years, many unmet needs remain for the more than 7.5 million adults in the U.S. living with the disease," said Dr. Jeffrey Stark, Head of Medical, U.S. Immunology, UCB, in a press release on November 14, 2023.
"With BIMZELX now available, patients and their healthcare providers have access to a treatment that has proven to deliver rapid, complete consistently, and maintained skin clearance from the first dose."
The approval of BIMZELX is supported by data from three Phase 3, multicenter, randomized, placebo, and/or active comparator-controlled trials, which evaluated the efficacy and safety of BIMZELX in 1,480 adults.
Patients treated with BIMZELX achieved superior levels of skin clearance at week 16 compared to those who received ustekinumab (ranked secondary endpoint, BE VIVID; p<0.0001), placebo (co-primary endpoint, BE READY and BE VIVID; p<0.0001) and adalimumab (co-primary endpoint, BE SURE; p<0.001), as measured by at least a 90 percent improvement in the Psoriasis Area & Severity Index (PASI 90) and an Investigator's Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). Ranked secondary endpoints included PASI 75 at week four and PASI 100 (complete skin clearance) at week 16.
Through BIMZELX Navigate, UCB offers tailored patient support to all those with moderate-to-severe plaque psoriasis upon receiving their BIMZELX prescription. Eligible patients living in the U.S. may enroll in BIMZELX Navigate at https://www.bimzelx.com/patient-support.
The AMA's What Doctors Wish Patients Knew™ series provides physicians a platform to share what they want patients to understand about today's healthcare headlines.
The AMA wrote living with psoriasis can be incredibly frustrating and challenging. Itchy, scaly skin can hinder daily activities, affect emotional well-being, and diminish the overall quality of life.
The encouraging news is that there are strategies to ease the challenges of living with psoriasis.

Nouscom recently announced the completion of its Series C equity financing, raising $72 million that will be used to continue advancing and expanding Nouscom's wholly-owned clinical pipeline to achieve multiple clinical value catalysts.
As of November 13, 2023, the funding proceeds will support the following initiatives:
Readout from Nouscom's ongoing randomized Phase 2 clinical trial for NOUS-209, an off-the-shelf vaccine targeting 209 shared neoantigens, in combination with pembrolizumab for the treatment of Mismatch Repair/Microsatellite Instable Metastatic Colorectal Cancer.
Final readout from the ongoing Phase 1b study and advancement of NOUS-209 monotherapy in Lynch Syndrome carriers investigating the potential to intercept, prevent, or delay cancer before it occurs. LS carriers have a genetic predisposition to and, consequently, a higher risk of developing certain cancers. Promising initial results from this study were reported on October 31, 2023.
Completion of a Phase 1b study evaluating NOUS-PEV, a personalized cancer immunotherapy, in combination with a checkpoint inhibitor in patients with advanced melanoma and entry into randomized Phase 2 trials in indications with high unmet medical needs.
Nouscom has also exclusively out-licensed VAC-85135, an off-the-shelf immunotherapy developed under a multi-project agreement, which is currently under evaluation in a Phase 1 clinical trial for the treatment of Myeloproliferative Neoplasms sponsored by Janssen Research & Development and Bristol Myers Squibb.
Dr. Marina Udier, Chief Executive Officer of Nouscom, commented in a press release, ".... This financing will allow us to further accelerate development across our wholly-owned clinical portfolio reporting multiple clinical trial readouts, including from our ongoing randomized Phase 2 clinical trial with NOUS-209."
"These Phase 2 data, if positive, have the potential to position Nouscom's neoantigen-based cancer vaccines amongst the most thrilling developments in the field."
According to a Review Article published by the journal Frontiers in Immunology in February 2023, Neoantigen vaccines are based on epitopes of antigenic parts of mutant proteins expressed in cancer cells. These highly immunogenic antigens may trigger the immune system to combat cancer cells.

While most chikungunya virus (CHIKV) cases have been reported throughout the Americas in 2023, this mosquito-transmitted virus is causing outbreaks in about 115 countries.
Approximately 320,000 CHIKV cases and over 340 related deaths have been reported worldwide this year.
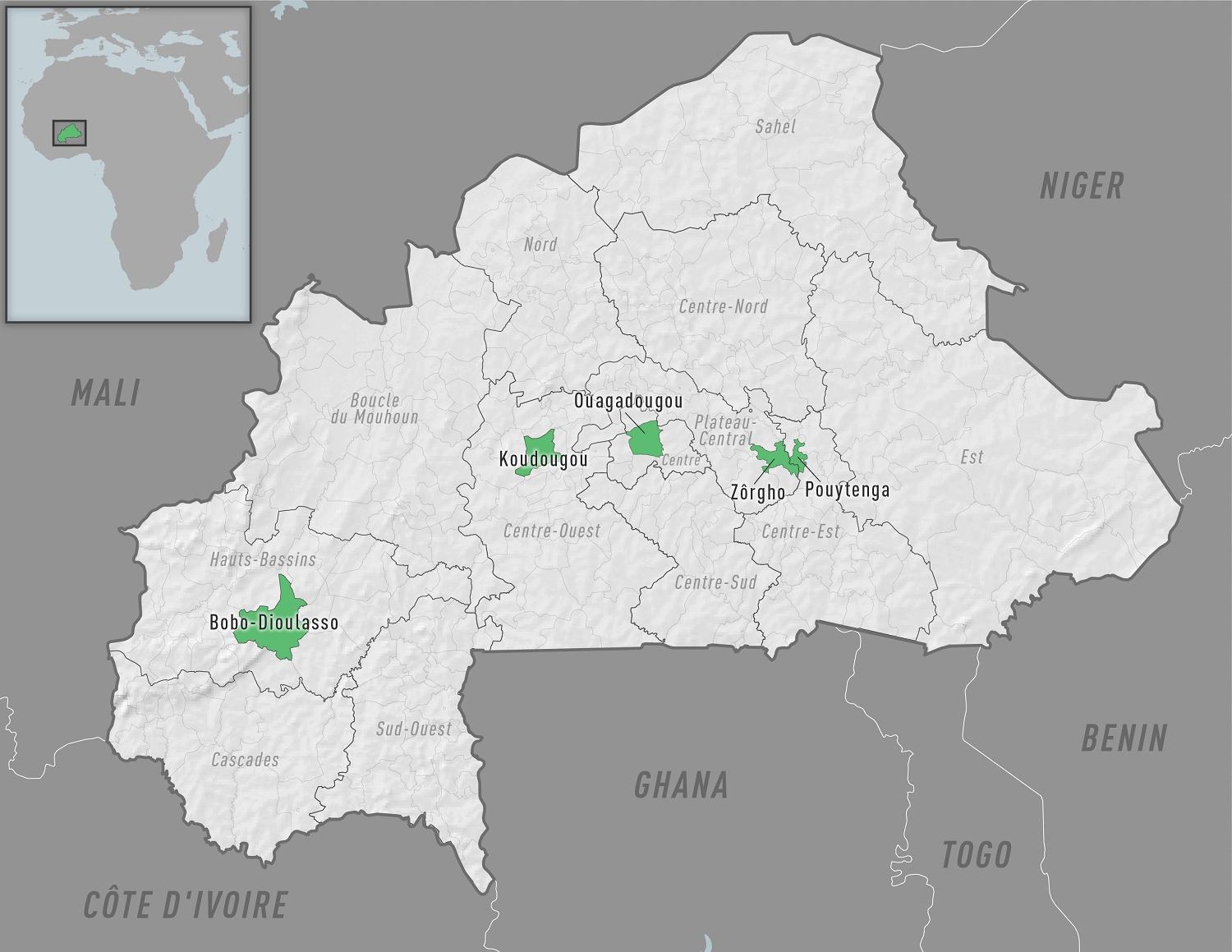
To alert international travelers, the U.S. Centers for Disease Control and Prevention (CDC) recently published a Level 2 - Practice Enhanced Precautions, Travel Health Advisory, regarding chikungunya outbreaks in Burkina Faso.
As of November 9, 2023, several districts have reported cases.
Burkina Faso Public Health Emergency Response Operations Center confirmed 89 chikungunya cases in Pouytenga in the Center-East region in September 2023.
Senegal, Burkina Faso's west African neighbor, has reported 210 chikungunya cases in 2023.
According to the CDC, chikungunya disease is caused by the virus and is spread to humans through mosquito bites.
If infected, you should seek medical care if you develop fever, joint pain, headache, muscle pain, joint swelling, or rash during or after travel.
If you are pregnant, reconsider travel to Burkina Faso, particularly if you are close to delivering your baby, says the CDC.
Mothers infected around the time of delivery can pass the virus to their baby before or during delivery. Newborns infected in this way are at risk for severe illness, including poor long-term outcomes.
Based on the U.S. Food and Drug Administration's recent approval of the IXCHIQ® Chikungunya Vaccine, Live (VLA1553), this disease can be prevented with a vaccine.
Other CHIKV vaccine candidates are conducting late-stage clinical trials in 2023.
Precedence Statistics today announced the global virology market size reached $2.6 billion in 2022 and is projected to reach $4.26 billion by 2032, expanding at a CAGR of 5.10%.
The U.S. virology market reached $690 million in 2022 and is projected to expand at a CAGR of 5.20%, reaching around $1.14 billion by 2032.
The virology market encompasses the study, diagnosis, treatment, and prevention of viral infections. It includes research, pharmaceuticals, diagnostic tests, and vaccines related to viruses like HIV, influenza, and hepatitis, which is a significant driver for the growth of the virology market.
The rapid mutation rates of many viruses pose hurdles for drug and vaccine development, necessitating ongoing research and adaptability.
The quest for effective vaccines to prevent viral diseases has also driven growth in this market. Government investments and public health initiatives have played a pivotal role in shaping the industry landscape.
The continuous evolution of vaccine technology, including mRNA and vector-based platforms, has broadened the scope of virology research.
Furthermore, the need for vaccine booster shots to combat emerging virus variants ensures a sustained demand for virology products.
Furthermore, the resumption of global travel contributes to the rapid spread of viruses, emphasizing the importance of virology in understanding, preventing, and managing infectious diseases worldwide.

