Search API
ADvantage Therapeutics, Inc. today announced that the first patient was enrolled in the Phase 2b clinical trial on its lead immunotherapy candidate, AD04™, for treating mild Alzheimer's disease (AD).
The study is authorized to be conducted in Austria, France, Poland, Bulgaria, and Slovakia and is expected to expand to Germany and the U.K. in the coming months.
AD04 has been used as an adjuvant in human and animal vaccination programs.
In a previous trial, AD04 serving as a control against another compound demonstrated a statistically significantly slower decline over other treatment groups in cognitive and quality-life clinical measures.
AD04 also showed a slower decline in hippocampal volume as a biomarker.
The Company believes that rather than being limited to a specific aspect of AD pathology, such as amyloid beta or tau, AD04 may address immunological mechanisms in the brain. And AD04 may function as an immunomodulator, stimulating and/or regulating the immune system to reduce AD pathology.
Dr. Andreas Winkler, MSc., principal investigator at Institut Neuromed, commented in a press release on November 27, 2023, "... AD04 represents a significant departure from approaches seen to date and one that may change our understanding of how to manage it as well as the disease itself."
Scientists do not yet fully understand what causes Alzheimer’s disease. Alzheimer’s is the most common type of dementia. About forty-four million people worldwide have Alzheimer's Disease, and it is the sixth leading cause of death in industrialized countries. The socio-economic burden of AD is enormous, says the U.S. CDC.
“We consider AD an autoimmune disease,” said Jeffrey Madden, CEO of ADvantage, in May 2023.
“And, instead of going after the consequences of AD, such as tau-protein and amyloid aggregates, we analyze immunological and neuroinflammatory events in specific parts of the brain. We believe these are causes of brain shrinkage and cognitive decline seen in AD.”
As of November 27, 2023, the U.S. FDA, the European Medicines Agency, and the U.K. have not approved an AD preventive vaccine candidate.

According to the UK Health Security Agency's weekly influenza report, the 2023-2024 flu season vaccination rates vary across age groups.
As of November 23, 2023, the provisional proportion of people in England who have received the 2023 to 2024 influenza vaccine in targeted groups is as follows:
- 75.4% in all aged 65 years and over, and is higher compared to the equivalent week in the 2022 to 2023 season
- 36.8% in those aged under 65 years in a clinical risk group, and is lower compared to the equivalent week in the 2022 to 2023 season
- 37.9% in all aged two years, and is comparable to the equivalent week in the 2022 to 2023 season
- 28.2% in all pregnant women is comparable to the equivalent week in the 2022 to 2023 season.
For week #47, multiple indicators show that flu activity has remained stable and within baseline levels in England this week.
Influenza positivity remained stable at 1.8% in week #46 compared to 1.8% in the previous week.
Hospital admissions, including intensive care unit and high dependency unit admissions, remained within baseline levels this week.
Children under five years continue to have the highest hospital admissions, but this has decreased this week to a rate of 0.92 per 100,000 population.
Global flu season news as of November 27, 2023, is posted at Precision Vax.

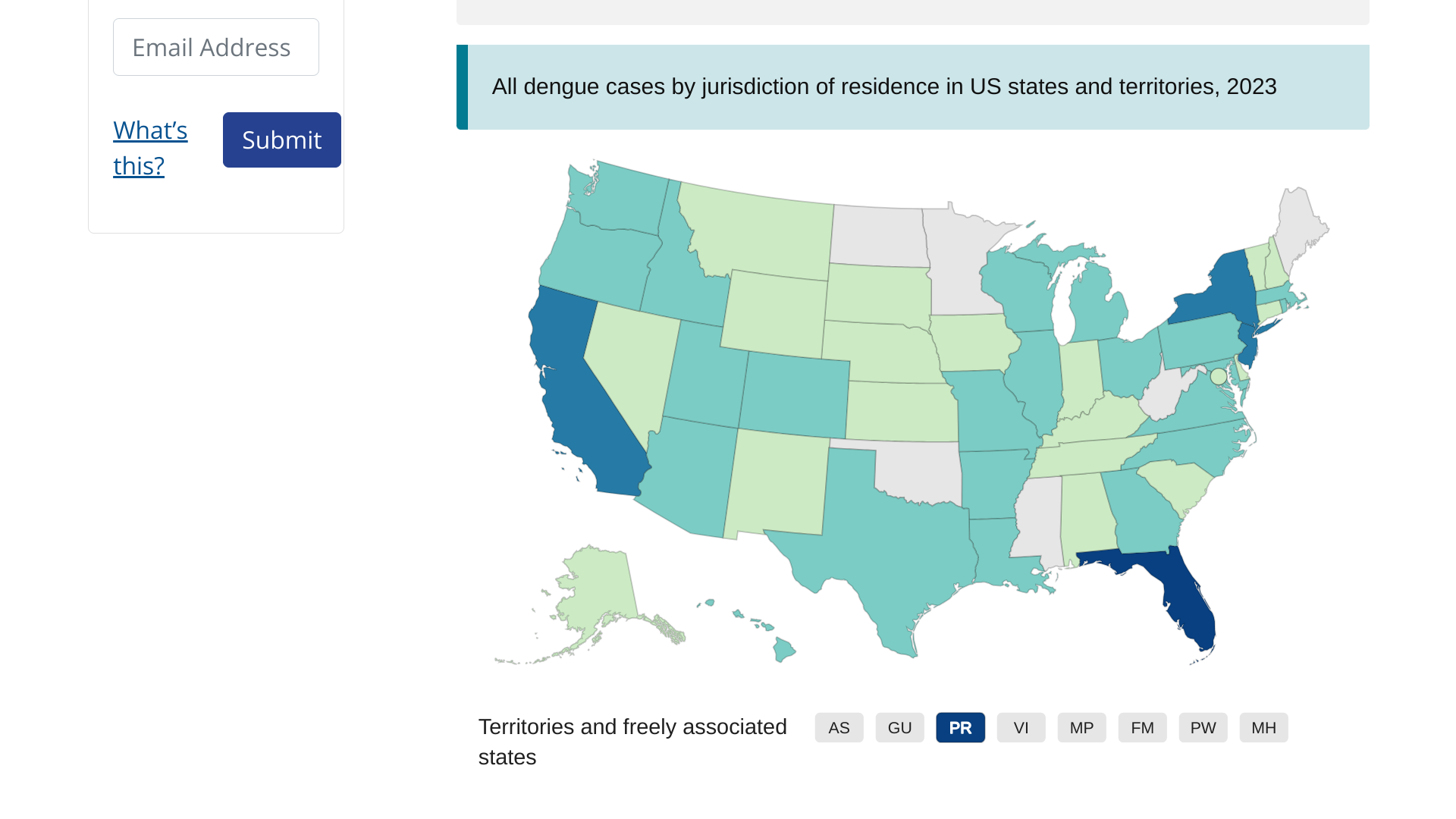
As the global dengue virus outbreak continues in about 80 countries with over 4.5 million dengue cases and over 4,000 dengue-related deaths, new data from Florida indicates there may be fewer cases this year.
Florida Health's Mosquito-Borne Disease Surveillance Report #46 confirmed 458 travel-associated dengue cases and 142 locally acquired dengue cases in 2023.
As of November 18, 2023, most of the travel-related dengue cases have been associated with visitors from Cuba (272).
And Miami-Dade Country has confirmed the most local dengue cases (133).
In 2022, Florida reported 903 travel-associated and 68 locally-acquired dengue cases.
From a prevention perspective, the U.S. FDA-approved Dengvaxia® vaccine is available but is seldom administered because of required diagnostic testing.
Dengvaxia (CYD-TDV) is a live attenuated tetravalent chimeric vaccine made using recombinant DNA technology.
Recently, the FDA extended Dengvaxia's approval to include children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic.
However, the vaccine is not approved for use in U.S. travelers who are visiting but not living in an area where dengue is common.
Endemic areas can include some U.S. territories and freely associated states.
A new dengue vaccine, QDENGA®, has recently been approved in various countries, without a pre-administration test requirement.
QDENGA (TAK-003) prevents dengue fever and/or severe dengue caused by any of the four serotypes.
In response to the ongoing Highly Pathogenic Avian Influenza (HPAI) outbreak in the western United States, ten vaccinated juvenile California condors will be released in the San Simeon mountains from November 28 to December 12, 2023.
The Condors were vaccinated with a killed, inactivated product conditionally licensed by the Center for Veterinary Biologics in 2016. The U.S. Fish and Wildlife Service has tested HAPI vaccine candidates on vultures in North Carolina for months.
This 'bird-flu' vaccine is designed to protect birds, not humans.
The U.S. government has already approved a bird flu vaccine for people and continues to invest in newer avian influenza vaccine candidates.
On November 23, 2023, John Fitzrandolph reported for The San Luis Obispo Tribune that 21 condors died from HAPI infections in Arizona earlier in 2023. Currently, the total population of condors is estimated at 347 birds in the wild spread across California, Arizona, and Baja, Mexico.
The California Condor Recovery Program is an international multi-entity effort to recover the endangered California condor.was last updated in November 2023.
The program aims to take steps toward recovery by establishing two geographically distinct, self-sustaining populations, each with 150 birds in the wild and at least 15 breeding pairs, with a third population of condors retained in captivity.
California condors, members of the vulture family Cathartidae, are one of the largest flying birds in North America, with a wingspan of nearly 10 feet.
In the wild, California condors may live up to 60 years. They mate for life and are attentive parents.
Avian influenza (Bird Flu) is a disease caused by influenza type A viruses that occur naturally among birds. The U.S. Centers for Disease Control and Prevention reported in June 2023, confirming the overall risk to human health associated with the ongoing HAPI outbreak in wild birds and poultry remains low.

The Denpasar City Agriculture Service recently announced villages/sub-districts in Bali Province to form a Rabies Alert Team to suppress cases of rabies transmission in the capital city of Bali Province.
It is estimated that there are 82,195 rabies-transmitting animals in Denpasar City, Indonesia, as of September 2023.
Announced on November 21, 2023, the Head of the Denpasar City Agriculture Service, A.A. Bayu Brahmasta, informed reporters, “The Rabies Alert Team will be tasked with providing education and outreach to the community.”
This news comes from TheBaliSun, which reported two additional residents who live near Keramas Beach were bitten by a dog that has gone on to test positive for rabies.
According to the U.S. CDC, dog rabies outbreaks have been active in Bali since 2008.
Bali is an often-visited destination, hosting over 4.8 million international visitors over the past year.
Each year throughout the world, rabies, a viral disease of mammals, kills approximately 50,000 people, primarily children. It is almost always spread by an animal bite but can also be spread when a rabid animal’s saliva gets directly into the eyes, nose, mouth, or broken skin.
The primary source of human infection worldwide is dogs. However, in the U.S., bats are the source of most rabies infections in 2023.
The CDC says that if your activities bring you into contact with animals, you should consider pre-exposure rabies vaccination, a multi-dose series given before departure overseas.
Even if you receive pre-exposure vaccination, you will still need immediate medical treatment if you are bitten or scratched by an animal.
The good news is human rabies is rare in the U.S., averaging about three cases annually since 2000.
In the U.S., rabies vaccines are available in 2023.

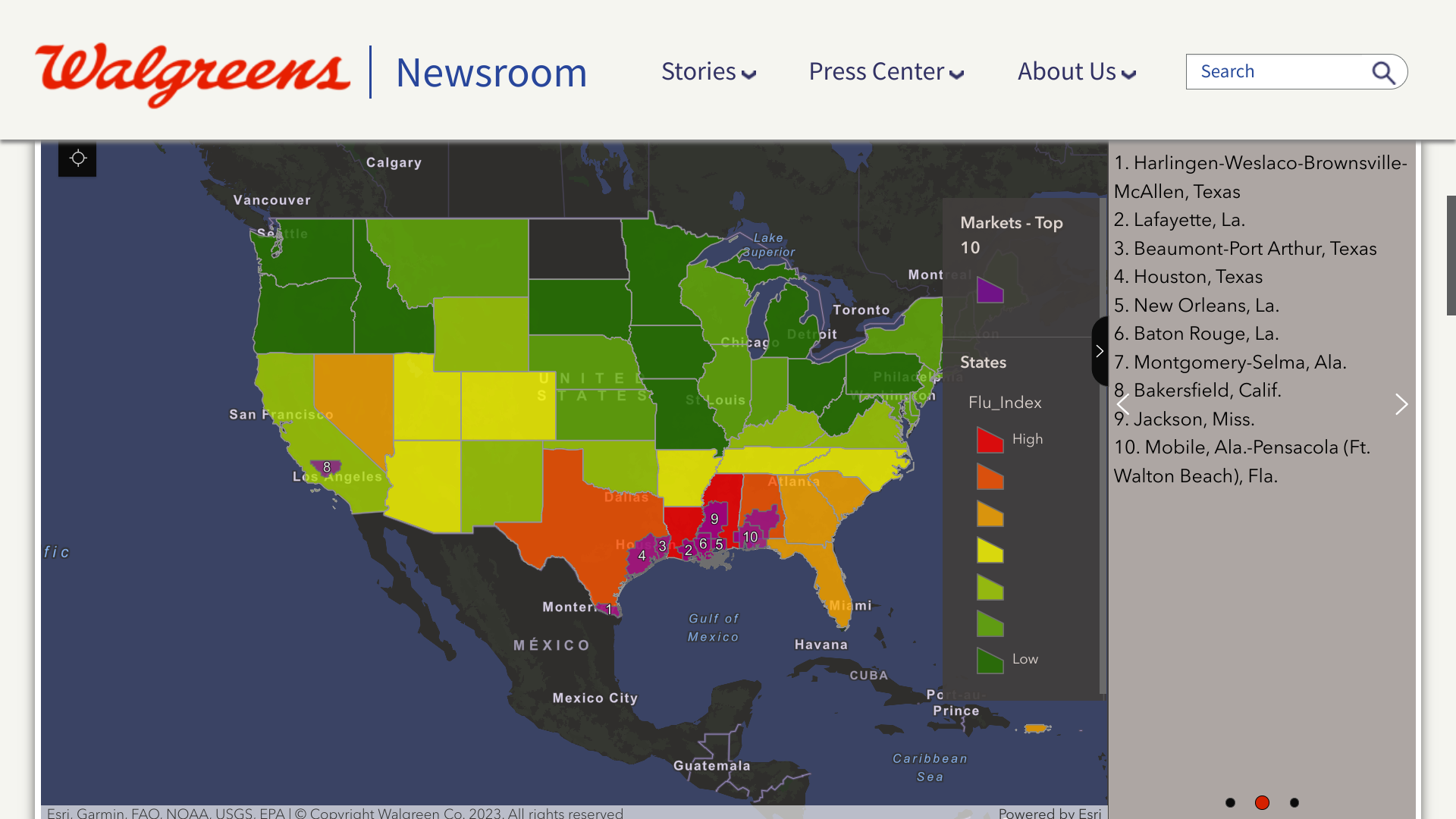
The Walgreens Flu Index® recently coded the top 10 United States cities experiencing the most influenza detections. The Index shows flu activity is 50% higher than last week.
As of November 18, 2023, the updated listing includes cities primarily located in the south-central U.S.:
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Lafayette, La.
- Beaumont-Port Arthur, Texas
- Houston, Texas
- New Orleans, La.
- Baton Rouge, La.
- Montgomery-Selma, Ala.
- Bakersfield, Calif.
- Jackson, Miss.
- Mobile, Ala.-Pensacola (Ft. Walton Beach), Fla.
While various flu shots are readily available at most clinics and pharmacies in October 2023, about 15% of individuals who received their flu shot at Walgreens were millennials, and only 9% were Gen Z.
The Walgreens Flu Index® is an online, interactive tool showing the most significant increases in flu activity week-over-week.
Shanghai Ark Biopharmaceutical Co., Ltd. (ArkBio) today announced that it had received IND approval from the National Medical Products Administration in China for a novel monoclonal antibody AK0610 that was bioengineered based on a respiratory syncytial virus (RSV) infection.
AK0610 specifically targets the RSV pre-F protein.
It demonstrated potent neutralizing effects against RSV both in vitro and in vivo. With a prolonged half-life, it holds promise as a next-generation long-acting antibody for RSV prevention.
Dr. Jim Wu, chairman and CEO of ArkBio, commented in a press release on November 23, 2023, "We are excited with the IND approval of AK0610 and its great potential in the field of RSV prevention."
"...We will strive to provide very needed RSV high-risk population and patients with efficacious prevention and treatment solutions."
The discovery and pre-clinical characterization have been published in hLife jointly by Professor George Fu Gao and his team from the Institute of Microbiology, Professor Zhengde Xie and his team from Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China, and ArkBio R&D team.
China has one of the highest rates of lower respiratory tract infection in children caused by RSV, accounting for 18-27% of all hospitalizations in children under five years old due to RSV infections.
ArkBio licensed AK0610 intellectual properties from the Institute of Microbiology, Chinese Academy of Sciences and Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China, followed by further engineering and optimization at ArkBio.
Currently, there is no approved drug for preventing RSV infection in China.
In the United States, RSV antibody passive immunization products have been U.S. FDA-approved since 1998.
Recently, AstraZeneca and Sanofi co-developed Beyfortus™ monoclonal antibody, which was approved by the FDA to protect infants through their first and second RSV season.

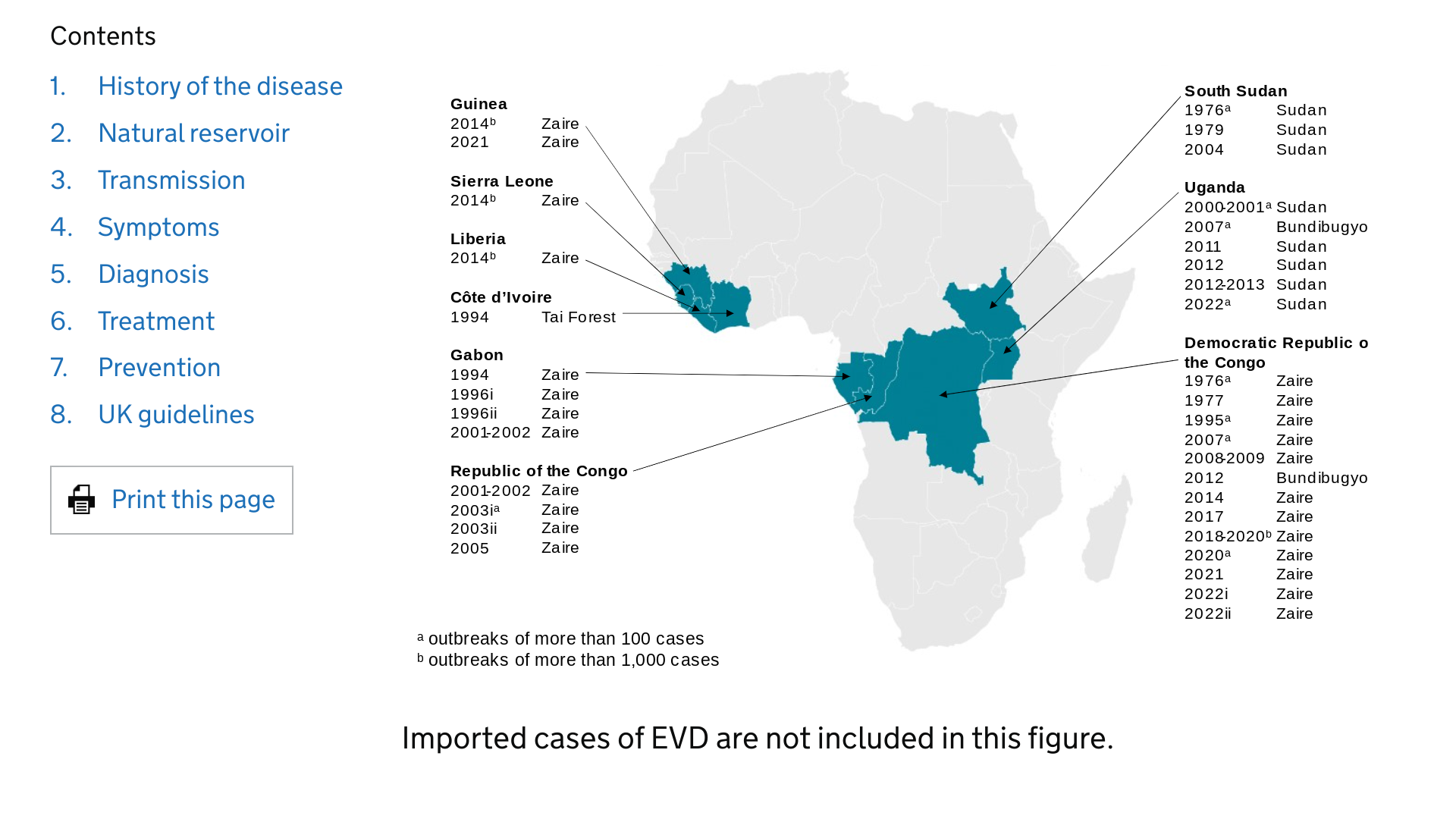
SK bioscience today announced it is partnering with Hilleman Laboratories Singapore to develop a low-cost, improved manufacturing process, second-generation Ebola-Zaire vaccine.
Currently, Ebola vaccines have been authorized and used in Africa since 2019.
On November 22, 2023, SK bioscience confirmed it will acquire unique expertise and know-how for the use of recombinant Vesicular Stomatitis Virus Vector (rVSV) technology platform in close collaboration with Hilleman Laboratories to potentially jointly develop other vaccines against a variety of viral infectious diseases.
Jaeyong Ahn, CEO of SK bioscience, commented in a press release, "Developing a vaccine to prevent viruses causing diseases with a high fatality rate, such as Ebola-Zaire, is essential for us to protect humanity."
"By cooperating with Hilleman Laboratories for a successful development of the second-generation Zaire Ebolavirus vaccine, we will contribute to overcoming the Ebola Zaire disease burden and expand our cooperation with global companies and institutions."
In 2014, the World Health Organization declared an international public health emergency during the Ebola outbreak and encouraged the development of the vaccine when the virus was spreading rapidly in West Africa.
Ebola Virus Disease is a rapidly progressive, severe, and transmissible hemorrhagic illness caused by infection with one of the Ebola Virus (EBOV) species. While there are six identified EBOV species, the Zaire Ebola virus strain has been the leading cause of outbreaks over the last 20 years.
Ever since the Ebola virus was first discovered in 1976, there have been multiple outbreaks resulting in significant loss of lives (50% mortality rate) and economic impact.