Search API
The U.S. Centers for Disease Control and Prevention (CDC) today announced very few seniors have received an approved vaccine that protects against Respiratory syncytial virus (RSV).
This common respiratory virus can become severe and require hospitalization.
As of December 8, 2023, the CDC confirmed the percentage of adults 60+ reporting receiving an RSV vaccine was 15.9%.
This CDC data indicates an uptake gap compared to a recent survey.
A recent poll by the University of Michigan's Institute for Healthcare Policy and Innovation revealed that 21% of seniors want an RSV vaccination in 2023.
Unlike previous RSV seasons, there are two RSV vaccines endorsed by government agencies, which are as follows:
AREXVY™ RSV vaccine is approved for adults aged 60 and above.
ABRYSVO™ RSVpreF RSV bivalent vaccine is from Pfizer Inc.
The U.S. Food and Drug Administration, the U.K.'s Medicines and Healthcare products Regulatory Agency, and the European Commission recommend adults 60 years and older and pregnant women receive a single dose of an RSV vaccine based on discussions with healthcare providers and under certain conditions.
Dr. Mandy Cohen, Director of the CDC, commented during a media interview on December 8, 2023, "What we know is that several viruses are circulating, but we also have tools to protect ourselves."
"And we're hoping more can do that before the Christmas holiday."
In the U.S., RSV vaccines are generally available at clinics and pharmacies.

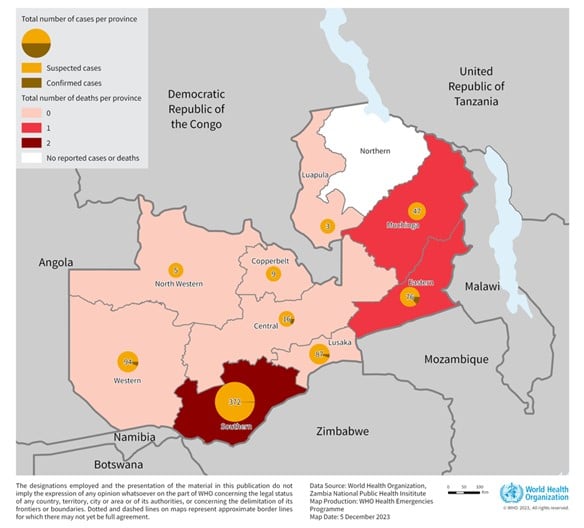
The ongoing anthrax outbreak in the Republic of Zambia has recently become a multi-country concern.
The World Health Organization (WHO today announced that as of November 20, 2023, 684 suspected human cases, including four deaths, have been reported in 2023.
This unprecedented anthrax outbreak marks the first significant occurrence spanning nine out of 10 country provinces. The last large-scale outbreak reported in Zambia occurred in 2011, with 511 suspected cases, wrote the WHO on December 8, 2023.
The risk of the outbreak spreading within Zambia is assessed to be high, and at the regional level is also considered high due to the frequent movement of both animals and people between Zambia and its neighboring countries, such as Angola, Botswana, the Democratic Republic of the Congo, Malawi, Mozambique, Namibia, Tanzania, Uganda, and Zimbabwe.
International travelers to anthrax-endemic countries should be aware of the current health risk, says the WHO. In 2021, about 554,000 tourists visited Zambia, formerly known as Northern Rhodesia, reported WorldData.
However, the WHO advises against implementing travel or trade restrictions with Zambia based on the current information on this event.
In addition to anthrax, the U.S. CDC has included Zambia in Travel Health Advisories in 2023 regarding measles and polio.
Humans usually acquire the infection after exposure to infected animals, carcasses, or animal products. More than 95% of human anthrax cases take the cutaneous form and result from handling infected carcasses or hides, hair, meat, or bones from such carcasses.
Anthrax is a zoonotic disease caused by Bacillus anthracis that typically affects ruminants (cows, sheep, and goats). The bacteria produce highly potent toxins responsible for the symptoms, causing a high lethality rate in the pulmonary form.
Humans can develop the disease from infected animals or through contaminated animal products. Hospitalization is required for all human cases identified. Vaccines are available for livestock.
However, humans have access to a limited supply.
From a prevention vaccination perspective, Emergent BioSolutions Inc. recently announced that the U.S. Biomedical Advanced Research and Development Authority awarded a $75 million contract option to acquire the newly licensed anthrax vaccine CYFENDUS™.
Deliveries of the two-dose vaccine are expected to begin in the U.S. in 2023 and be completed in the late first quarter of 2024. These vaccines are unavailable to the general public.
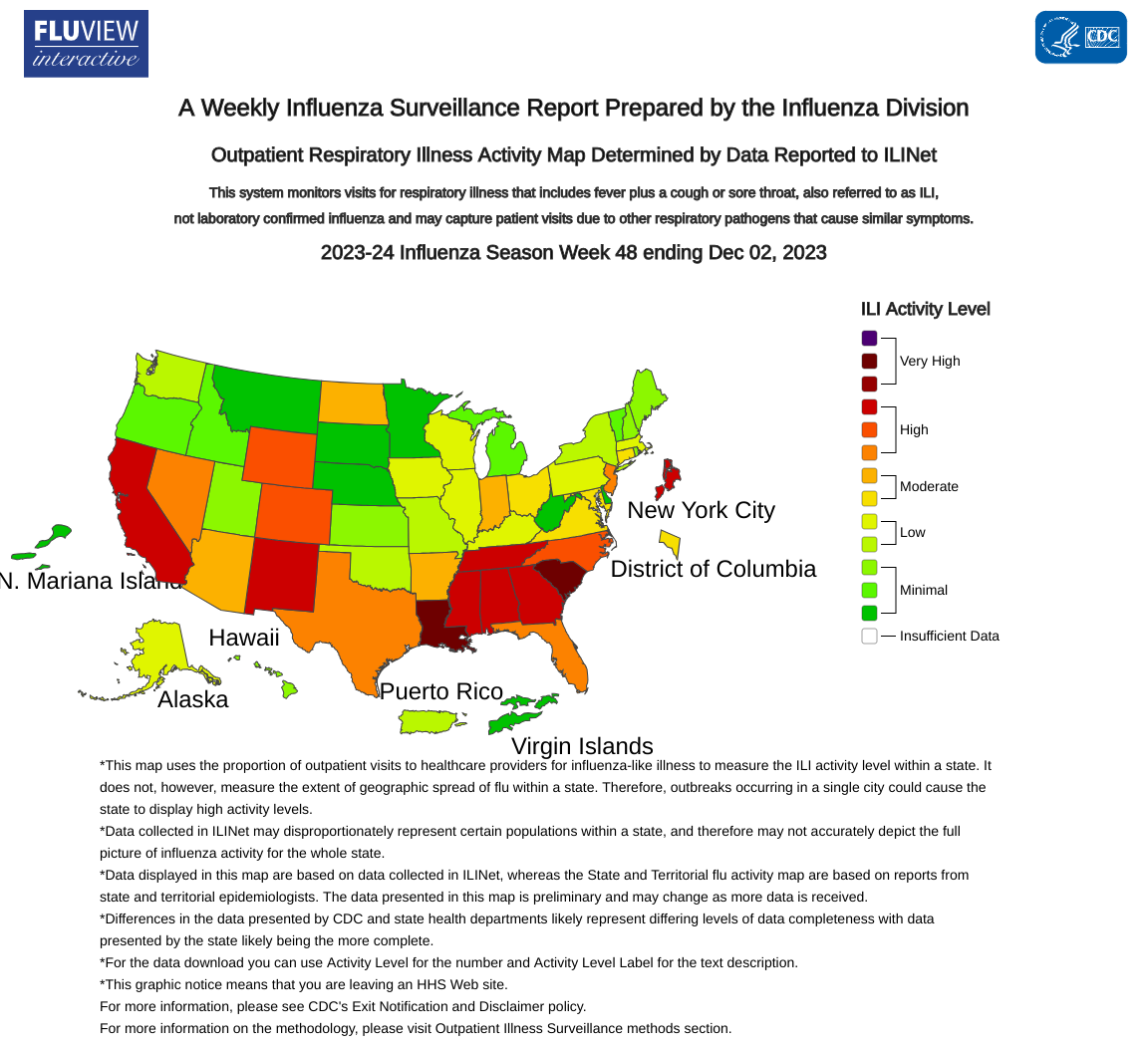
The U.S. Centers for Disease Control and Prevention (CDC) today announced seasonal flu activity continues to increase in most parts of the country, with the southeast and south-central areas of the country reporting the highest levels of activity.
As of December 8, 2023, outpatient respiratory illness is above baseline nationally for the fifth week and is at or above baseline in all 10 HHS Regions.
Furthermore, the National Center for Health Statistics (NCHS) Mortality Surveillance data available on December 7, 2023, indicates that 0.2% of the deaths (48) that occurred during Week #48 were due to influenza.
Last week, in #47, there were 61 flu-related deaths and 2,373 pneumonia-related deaths.
Specifically, there were four influenza-associated pediatric deaths reported during Week #48, bringing the 2023-2024 season total to 12 pediatric deaths.
Last flu season, about 182 children died from influenza.
From a prevention perspective, the CDC recently confirmed that over 151 million flu shots had already been distributed in the U.S.
These influenza vaccines are generally available at clinics and pharmacies,
The journal Nature Medicine recently asked researchers to name their top clinical trial picks for 2024, from base editing and a vaccine against human immunodeficiency virus (HIV).
On December 7, 2023, Carrie Arnold and Paul Webster wrote ...with so many rollercoaster years since the start of the pandemic, it is impossible to predict exactly what the biomedical world will deliver in 2024.
Experts identified which trials will likely have an outsized impact on medicine in 2024.
One expert, Carey Hwang, a senior vice president and head of clinical research at Vir Biotechnologycommented, highlighted VIR-1388, a cytomegalovirus (CMV) vector vaccine that induces strong, unique, and sustained T cell responses that can potentially prevent the acquisition of HIV.
The HIV Vaccine Trials Network is conducting a clinical trial at ten sites in the U.S. and two sites in South Africa, with support from the U.S. National Institute of Allergy and Infectious Diseases and the Bill & Melinda Gates Foundation.
From a public health perspective, having a vaccine against HIV would have a tremendous impact, commented Hwang.
As of December 8, 2023, the U.S. FDA has not approved any HIV vaccine candidates.
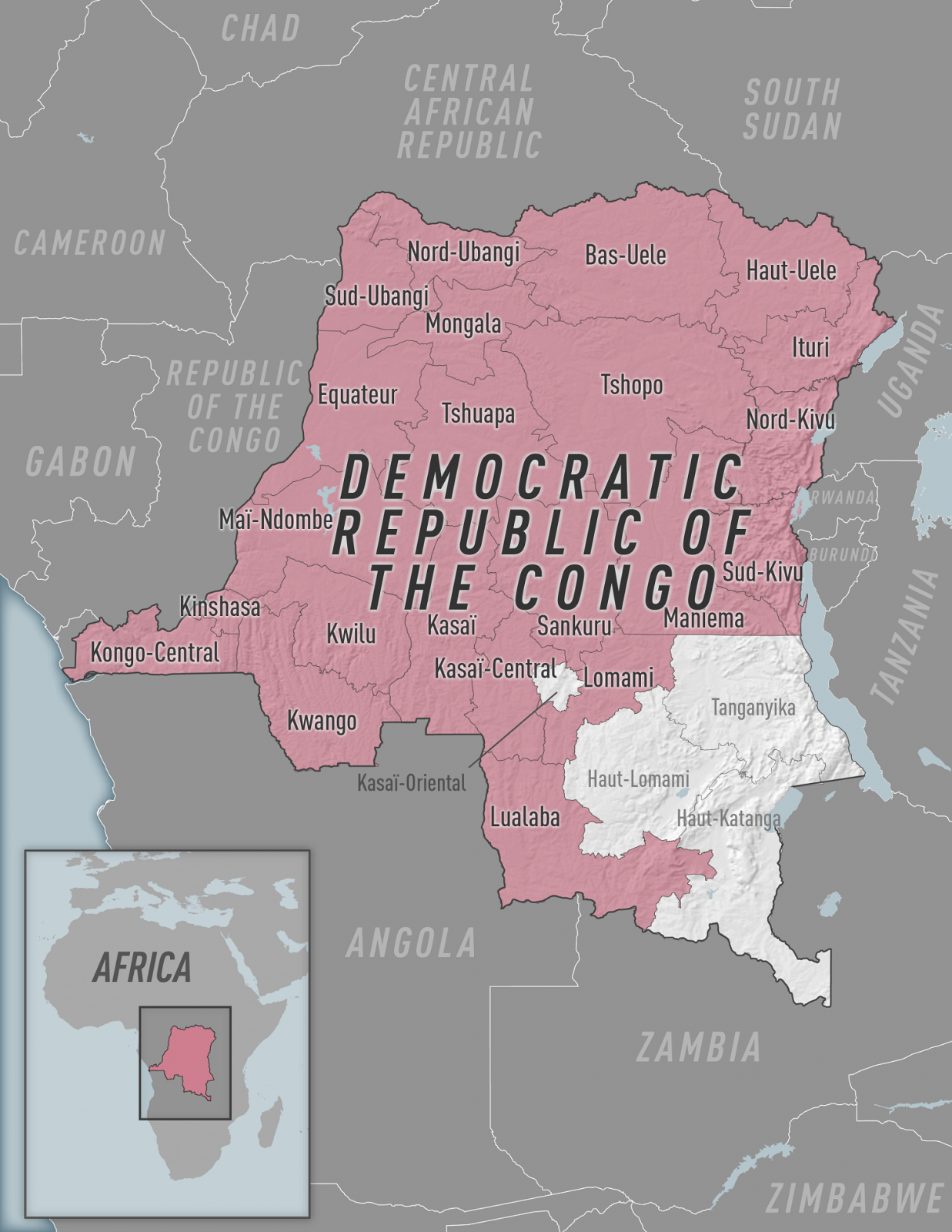
The U.S. Centers for Disease Control and Prevention (CDC) today announced a Health Alert Network (HAN) Health Advisory about the occurrence, geographic spread, and sexually associated human-to-human transmission of Clade I Monkeypox virus (MPXV) in the Democratic Republic of the Congo (DRC).
Since January 2023, the DRC has reported 12,569 suspected mpox cases and 581 related deaths from 22 regions.
The new HAN says cases of Clade I MPXV have not been reported in the United States as of December 7, 2023. The global outbreak of Clade II MPXV was initially reported in May 2022.
However, clinicians should be aware of the possibility of Clade I MPXV in travelers who have been in DRC.
Third-party data indicate that the number of tourists arriving in the DRC was about 460,880 in 2021.
The CDC recently issued a Travel Health Notice (Level 2 - Practice Enhanced Precautions) for people traveling to DRC. Furthermore, there are no direct commercial passenger flights from DRC to the U.S. as of December 2023.
U.S. FDA-approved vaccines (JYNNEOS, ACAM2000) are expected to be effective for both Clade I and II MPXV infections.
Vaccination or prior MPXV infection should provide antibodies that will provide cross-protection to other orthopoxviruses, including Clade I MPXV, says the CDC.
However, clinical verification is under review.
The CDC recommends clinicians encourage vaccination for eligible patients.
Eligible patients who have only received one dose of Bavarian Nordic JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine, which is based on a live, attenuated vaccinia virus, should receive the second dose as soon as possible, regardless of the time that has elapsed since the first dose.
Mpox vaccines have limited availability in the U.S.
Furthermore, clinicians should notify their state health department if they have a patient with mpox-like symptoms and should submit lesion specimens for clade-specific testing for these patients.
The World Health Organization (WHO) announced today's preliminary 2023 data, indicating that the number of cholera cases reported this year has surpassed that recorded in 2022.
As of December 7, 2023, over 610,000 cholera cases and 3,500 related deaths have been reported by 29 countries.
The WHO confirmed that given the extensive number of outbreaks, their widespread distribution, and the current shortage of oral cholera vaccines (OCV), the WHO continues to assess the risk at the global level as very high.
At the end of November 2023, around 65 million OCV doses have been requested, with 45% being approved and allocated to 12 countries.
The global OCV stockpile is 4.5 million doses, available but not yet allocated.
There are three WHO pre-qualified OCVs: Dukoral®, Shanchol™, and Euvichol®.
In the current outbreak context, only one-dose courses have been validated and implemented in these reactive campaigns, says the WHO.
Since the beginning of 2023, 24 reactive vaccination campaigns have been implemented in 12 countries facing cholera outbreaks: Ethiopia (4), Mozambique (4), Kenya (3), Somalia (2), Northwest Syria (2), Cameroon (2), Sudan (2), the Dominican Republic (1), Democratic Republic of Congo (DRC) (1), Haiti (1), Malawi (1), and Zambia (1).
From an OCV availability perspective, the U.S. Food and Drug Administration recommends OCV for specific conditions in countries undergoing outbreaks.
However, vaccination against cholera is not generally recommended because most U.S. travelers do not visit cholera outbreaks.
In August 2023, the U.S. Centers for Disease Control and Prevention published Cholera Vaccine Recommendations, highlighting the Vaxchora® vaccine.
All OCVs require two doses for complete protection against cholera for up to three years, while a single dose provides short-term protection.

Anixa Biosciences, Inc. today announced new and updated positive results from the Phase 1 clinical trial of its breast cancer vaccine.
The data were presented by G. Thomas Budd, M.D., a staff physician at Cleveland Clinic Cancer Institute and principal investigator of the study, in a poster entitled "Phase I Trial of alpha-lactalbumin vaccine in high-risk operable triple negative breast cancer (TNBC) and patients at high genetic risk for TNBC."
Patients who had been curatively treated for TNBC received three vaccinations given once every two weeks. IFNγ and IL-17, which are T cell immune response indicators (cellular immunity), and antibody production (B cell humoral immunity) were measured to evaluate the vaccination effect.
Data from the 16 patients treated to date showed that:
Most patients developed ELISpot (T-cell) responses that met the rigorous protocol-specified definition of an immune response, with a measurable but lesser magnitude of response noted in the remaining patients.
12 (75%) of the women had antigen-specific IFNγ and/or IL-17 ELISpot responses at all dose levels, while ELISA antibody responses at Dose Level 2 and higher.
A statistically significant (P = 0.03) increase in IFNγ over baseline (Day 0) was observed by Day 56, while a significant (P = 0.0001) increase in IL-17 over baseline was observed by Day 14.
Among the doses studied, Dose Level 1 (10 mcg α-lactalbumin/10 mcg zymosan) was determined to be a usable immunologic dose and the maximum tolerated dose (MTD).
No significant side effects were observed at the MTD besides irritation at the injection sites. No myalgias, flu-like symptoms, or aberrant laboratory values were noted.
Anixa and Cleveland Clinic plan to investigate additional intermediate dose levels and continue studying the vaccine's safety and immunologic effects in two additional patient cohorts.
The first cohort, which opened for enrollment in August 2023, is evaluating the combination of the Company's breast cancer vaccine with Keytruda® (pembrolizumab) in post-operative patients found to have residual disease following neoadjuvant chemo-immunotherapy.
The second cohort will investigate the safety and immunologic effects of the vaccine in patients who are BRCA1, BRCA2, or PALB2 mutation-positive and are planning prophylactic risk-reducing mastectomies.
"The data from our Phase 1 trial has exceeded our expectations, and we are pleased with our progress. This vaccine is designed to direct the immune system to destroy TNBC cancer cells through a mechanism that has never previously been utilized for cancer vaccine development," stated Dr. Amit Kumar, Chairman and CEO of Anixa Biosciences, in a press release on December 7, 2023.
"We look forward to reviewing additional data as the trial continues to completion, and we are in the planning stages of the Phase 2/3 studies of this vaccine."
"Our goal is to initially evaluate the vaccine's ability to prevent recurrence of cancer in survivors and continue with extension studies to eventually determine its effectiveness in preventing the initial onset of TNBC."
Anixa is the exclusive worldwide licensee of the novel breast cancer vaccine technology invented at Cleveland Clinic, the site of the Phase 1 trial. The U.S. Department of Defense grant was made directly to the Cleveland Clinic.

As the United States heads into the winter months that typically coincide with peak respiratory illness season, Walgreens provides in-store and at-home testing and treatment options to help everyone stay healthy and feel better faster if they are experiencing symptoms.
"Respiratory illness activity and hospitalizations are picking up in many parts of the U.S., and these numbers are likely to continue increasing in the coming months," said Anita Patel, PharmD, Vice President of Pharmacy Service Development at Walgreens, in a press release on December 7, 2023.
"In addition to staying up to date on your vaccinations, getting tested, seeking treatment promptly, and practicing good respiratory etiquette are all important steps to protect yourself and your loved ones this winter, especially if you are feeling sick or planning to travel and gather for the holidays."
Walgreens stated testing is the best way to know if you have a specific respiratory virus so you can take appropriate precautions and get the proper relief or treatment immediately.
With influenza, RSV, and COVID-19 viruses circulating, visiting a local pharmacist in 2023 may be just what the doctor ordered.
Flu and RSV vaccines are covered by most insurance plans with a $0 copay and by Medicare and Medicaid in certain states.
Dr. Patel added, "People are increasingly relying on pharmacies as a one-stop destination for these services and deepening their relationships with their community pharmacists, who work tirelessly to provide the care and information they need all season long."
"As the nature of respiratory illness season continues to evolve post-pandemic, we remain focused on being a trusted partner in keeping our communities healthy."
As of December 2, 2023, the Walgreens Flu Index listed the top three states indicating influenza outbreaks:
- Louisiana
- Mississippi
- Texas
With nearly 9,000 retail locations across America, Puerto Rico, and the U.S. Virgin Islands, Walgreens is proud to be a neighborhood health destination serving almost 10 million customers daily.
Various types of flu shots are available as of December 7, 2023.

The approval of yet another RNA-based vaccine might not seem momentous, wrote Elie Dolgin in an article published by the journal Nature.
But the endorsement last week by Japanese authorities of a jab against the SARS-CoV-2 coronavirus constructed using a form of RNA that can make copies of itself inside cells — the first 'self-amplifying' RNA granted full regulatory approval anywhere in the world — marks a pivotal advance.
The December 6, 2023, article is posted at this link.
In late November 2023, CSL and Arcturus Therapeutics announced that Japan's Ministry of Health, Labor, and Welfare approved ARCT-154, a self-amplifying Messenger RNA COVID-19 vaccine for initial vaccination and booster for adults 18 years and older.

