Search API
Eisai Co., Ltd. and Biogen Inc. today announced that humanized anti-soluble aggregated amyloid-beta (Aβ) monoclonal antibody LEQEMBI® Intravenous Infusion (200 mg, 500mg, lecanemab) is scheduled to launch in Japan on December 20, 2023.
LEQEMBI received manufacturing and marketing approval for the indication of slowing progression of mild cognitive impairment and mild dementia due to Alzheimer's disease (AD) in Japan on September 25, 2023.
The pending launch in Japan marks the second country to have LEQEMBI on the market, following the U.S. Food and Drug Administration (FDA) approval in July 2023.
In the U.S., treatment with LEQEMBI should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials, says the FDA.
"The availability of LEQEMBI opens a new era in the treatment of AD, potentially giving patients and their families additional valuable time together and further positions Japan as a leader in caring for an elderly population," said Christopher A. Viehbacher, President and Chief Executive Officer of Biogen, in a press release on December 13, 2023.
"We will work alongside Eisai to engage the medical community and support the patient journey, especially early diagnosis, as mounting evidence suggests early intervention may provide a greater impact on disease progression."

More than 20 anthrax-related deaths have been reported in five countries in Africa since the start of 2023.
As of December 11, 2023, a total of 1,166 suspected and 37 confirmed cases have been recorded in Kenya, Malawi, Uganda, Zambia, and Zimbabwe, according to data reported to the World Health Organization (WHO).
Of the five countries, Zambia is witnessing its largest anthrax outbreak since 2011, with 25 confirmed cases and four deaths. Only sporadic cases have previously been reported in animals and humans in the country.
Over 400,000 vaccine doses have been earmarked for high-risk districts in Zambia's western province.
Annually, human anthrax infections are the highest in Africa, the Middle East, and Central and South Asia, says the WHO
"To end these outbreaks, we must break the infection cycle by first preventing animal disease. We are supporting the ongoing national outbreak control efforts by providing expertise as well as reinforcing collaboration with partner agencies for a common approach to safeguard human and animal health," said Dr Matshidiso Moeti, WHO Regional Director for Africa, in a press release.
Humans become infected with anthrax, a zoonotic disease, through contact with disease carrying animal carcasses or exposure to contaminated animal products. Rare person-to-person transmissions have been reported with cutaneous anthrax, which accounts for more than 95% of human cases worldwide.
Cutaneous anthrax usually develops 1–7 days after exposure, but incubation periods up to 17 days have been reported.
A study published by the journal Nature Biology estimated that 1.83 billion people (95% credible interval (CI): 0.59–4.16 billion) live within regions of anthrax risk, but most of that population faces little occupational exposure.
Individuals potentially exposed to anthrax spores may be provided with prophylactic treatment. Anthrax responds well to antibiotics, says the WHO.
In the United States, Emergent BioSolutions Inc.'s CYFENDUS™ (Anthrax Vaccine Adsorbed, Adjuvanted) was approved on July 20, 2023, as a two-dose anthrax vaccine for Post-Exposure Prophylaxis use.
However, the U.S. CDC says vaccination against anthrax is not recommended for travelers and is not available for civilian travelers.

GSK plc today announced the recipients of the inaugural grant program of the COiMMUNITY Initiative, a multipronged effort to support the design of a more systematic, collaborative and equitable approach to helping increase adult immunization rates in the United States.
Grant recipients include but are not limited to the American Lung Association, American Pharmacists Association (APhA), APhA Foundation, Asian Health Coalition, Global Health Living Foundation, Immunize Kansas Coalition, Pharmacy Quality Alliance, and The Arizona Partnership for Immunization.
Each grant-funded project receives between $50,000 and $175,000 out of a total of $1 million to help address long-standing barriers to adult immunization in the U.S.
Rob Truckenmiller, Senior Vice President, Head of U.S. Vaccines, GSK, commented in a press release on December 12, 2023, "Getting ahead of vaccine-preventable disease starts by investing in organizations who know their communities best, and I can't think of a more deserving group of recipients for our first-ever COiMMUNITY Initiative grants."
"With these grants, our goal is to support innovative ways to encourage adult vaccination and help mitigate health inequities at both the local and national levels."
The organizations that received funding submitted proposals that demonstrated innovative, ambitious, and actionable ideas for boosting adult vaccination, such as building vaccine confidence among underserved patient populations through trusted community partners, creating engaging multilingual educational materials, and developing resources to support pharmacists and local health departments.
Through the COiMMUNITY Initiative, GSK will continue to build on its commitment to help support long-lasting solutions to address adult immunization challenges through ongoing investments to empower stakeholders with actionable data, models, and best practices in adult vaccine confidence and delivery.

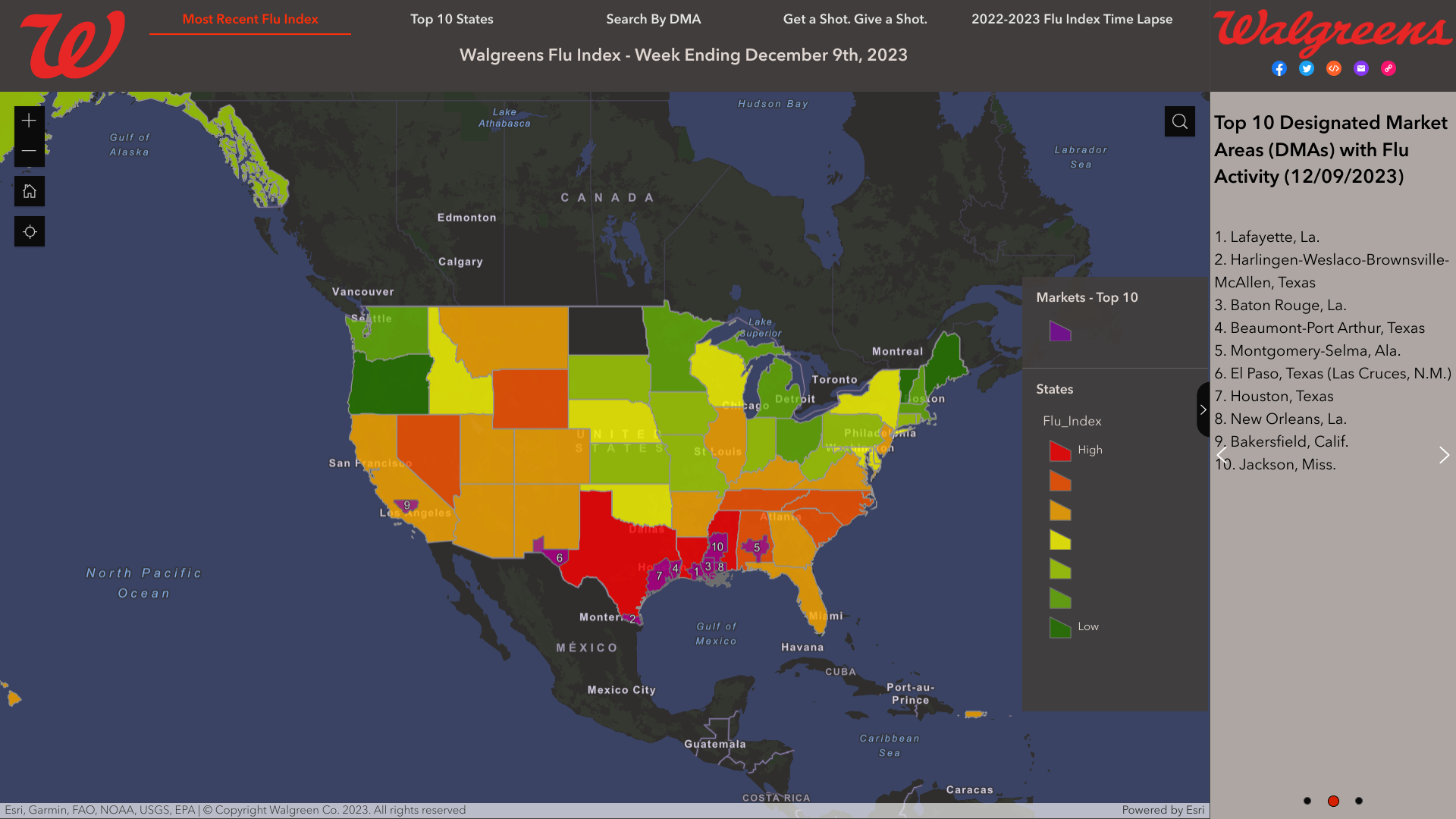
As national influenza rates increased post-Thanksgiving, according to the Walgreens Flu Index®'s latest report, the state of Texas is leading the nation in influenza activity.
As of December 9, 2023, the Index identified the leading cities using retail prescription data for antiviral medications used to treat influenza across Walgreens locations nationwide.
- Lafayette, La.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Baton Rouge, La.
- Beaumont-Port Arthur, Texas
- Montgomery-Selma, Ala.
- El Paso, Texas (Las Cruces, N.M.)
- Houston, Texas
Anita Patel, PharmD, Vice President of Pharmacy Service Development at Walgreens, commented in a press release on December 7, 2023, ".... practicing good respiratory etiquette are all important steps to protect yourself and your loved ones this winter, especially if you are feeling sick or planning to travel and gather for the holidays."
Testing is the best way to know for sure if you have a specific respiratory virus so you can take appropriate precautions and get the proper relief or treatment immediately.
Once you know your test results, your pharmacist can help you determine the best next steps and get the appropriate treatment as soon as possible, whether that's a prescription medicine or over-the-counter essentials to manage your symptoms, says Walgreens.
Furthermore, the U.S. CDC recommends getting an annual flu shot before gathering with friends and family this holiday season.
As of December 2, 2023, over 152 million flu vaccines (nasal, cell-based, egg-based) have been distributed in the U.S. and are available at most clinics and pharmacies.
The Walgreens Flu Index is not intended to illustrate levels or severity of flu activity but rather to illustrate which populations are experiencing the highest incidence of influenza.
Icosavax, Inc. today announced it had entered into a definitive agreement under which AstraZeneca would purchase the company Phase 2 study of IVX-A12, a combination virus-like particle (VLP) vaccine candidate targeting both respiratory syncytial virus (RSV) and human metapneumovirus (hMPV).
There are currently no treatments or preventative therapies for hMPV. Adults with hMPV infection may have viral pneumonia, worsening asthma, or COPD symptoms. And there are no combination vaccines for RSV.
Announced on December 11, 2023, Iskra Reic, Executive Vice President, Vaccines & Immune Therapies, AstraZeneca, commented in a press release, "This VLP vaccine technology has the potential to transform prevention against severe infectious diseases, including RSV and hMPV."
"With the addition of Icosavax's Phase III-ready lead asset to our late-stage pipeline, we will have a differentiated, advanced investigational vaccine and a platform for further development of combination vaccines against respiratory viruses."
"This aligns with our strategy to deliver a portfolio of therapies to address high unmet needs in infectious diseases and our ambition to protect the most vulnerable patients who have a high risk of severe outcomes."
Separately, Icosavax announced positive topline interim results from its Phase 2 clinical trial of IVX-A12 against RSV and hMPV in older adults.
IVX-A12 induced robust immune responses against both RSV and hMPV at Day 28 across both formulations with and without adjuvant.
"We're delighted to announce positive topline interim data from our Phase 2 trial of IVX-A12, our potential first-in-class combination vaccine candidate against RSV and hMPV," said Adam Simpson, Chief Executive Officer of Icosavax, in a press release.
"We believe that IVX-A12 has the potential to address a significant unmet need and, as the furthest advanced RSV and hMPV combination vaccine in the clinic, to build on an emerging, large market opportunity."
The ongoing Phase 2 clinical trial of IVX-A12 is a randomized, observer-blinded, placebo-controlled, multicenter trial designed to evaluate the safety and immunogenicity of a single dose of RSV and hMPV combination VLP vaccine IVX-A12, with and without CSL Seqirus' proprietary adjuvant MF59®.
Regarding the proposed acquisition, the upfront cash portion of the consideration represents an equity value of approximately $838 million, a 43% premium over Icosavax's closing market price on December 11, 2023, and a 73% premium to Icosavax's volume-weighted average price for the preceding 60 trading days.
Combined, the upfront and maximum potential contingent value payments represent, if achieved, an equity value of approximately $1.1 billion, a 91% premium over Icosavax's closing market price on December 11, 2023, and a 130% premium to Icosavax's volume-weighted average price for the preceding 60 trading days.

Alzamend Neuro, Inc. today announced receipt of a "Study May Proceed" letter from the U.S. Food and Drug Administration ("FDA") for the initiation of study AL001-PTSD01, a Phase IIA clinical study of AL001 for the treatment of patients with post-traumatic stress disorder ("PTSD").
AL001 is a novel lithium-delivery system that can potentially deliver the benefits of marketed lithium salts while mitigating or avoiding currently experienced toxicities associated with lithium.
"Although lithium does not have an FDA-approved indication for PTSD, it has been prescribed off-label for this purpose for decades," said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on December 11, 2023.
"If we can develop a next-generation lithium product (AL001) that would not routinely require therapeutic drug monitoring, it would constitute a major improvement over current lithium-based treatments and positively impact the 9 million Americans afflicted with PTSD."
"We are advancing the process and expect that the first patient will be dosed in the first quarter of 2024."
AL001 is designed to favorably distribute lithium in the brain resulting in lower exposure to other body organs and an improved safety profile compared to currently marketed lithium salts.
This can serve to mitigate or obviate the disadvantageously low ceiling for toxicity of marketed lithium salts that have limited their usefulness to patients and prescribers.
PTSD is a mental and behavioral disorder that can develop because of exposure to a traumatic event, such as sexual assault, warfare, traffic collisions, child abuse, domestic violence, or other threats to a person's life.
According to the U.S. NIH, about 3.6% of adults in the U.S. have PTSD in a given year, and 9% of people develop it at some point in their life. Worldwide, rates for PTSG in a given year are between 0.5% and 1% of the population.

Merck and Moderna, Inc. today announced the initiation of a pivotal Phase 3 randomized clinical trial (INTerpath-002) evaluating V940 (mRNA-4157), an investigational individualized neoantigen therapy, in combination with KEYTRUDA®, Merck’s anti-PD-1 therapy, as adjuvant treatment in patients with completely resected (R0) Stage II, IIIA or IIIB (with nodal involvement) non-small cell lung cancer.
Global recruitment of the INTerpath-002 has begun, and the first patients enrolled in Australia.
“As lung cancer is the leading cause of cancer death worldwide, there is a need for continued scientific advancements to help fight this disease at earlier stages when patients have the best chance for better outcomes,” said Dr. Marjorie Green, senior vice president and head of late-stage oncology, global clinical development, Merck Research Laboratories, in a press release on December 11, 2023.
“By combining KEYTRUDA with V940 (mRNA-4157), a promising new modality, we are researching innovative new approaches for earlier stage non-small cell lung cancer.”
As previously announced, in addition to INTerpath-002, the combination of V940 (mRNA-4157) plus KEYTRUDA is being investigated in INTerpath-001, a global, randomized, double-blind, placebo- and active-comparator-controlled Phase 3 trial evaluating patients with resected high-risk (Stage IIB-IV) melanoma.
INTerpath-001 is actively screening in 14 countries, representing 38 sites.
The companies confirmed they plan to expand the comprehensive clinical development program for V940 (mRNA-4157) to additional tumor types.

The U.S. Centers for Disease Control and Prevention (CDC) continues to publish Trave Health Notices regarding diphtheria outbreaks in various countries in 2023.
On December 7, 2023, the CDC posted a Level 2 - Practice Enhanced Precautions notice regarding an outbreak of diphtheria in several districts in Guinea, which is located in western Africa.
Diphtheria is a severe infection caused by strains of Corynebacterium diphtheriae bacteria that make a toxin. The toxin can cause people to get very sick. Diphtheria bacteria spread from person to person through respiratory droplets, like from coughing or sneezing.
People can also get sick from touching open sores or ulcers of people ill with diphtheria, according to the CDC.
Diphtheria is a vaccine-preventable disease.
Unfortunately, an estimated 16% of children worldwide had no or incomplete vaccination coverage.
The U.S. CDC says most travelers visiting outbreak areas should receive an age-appropriate dose of diphtheria toxoid-containing vaccine if they are not fully vaccinated or have not received a booster dose within five years before departure.
There are 11 vaccines available for use to help protect against diphtheria in 2023. Diphtheria and other travel vaccines are offered at many clinics and pharmacies in the U.S.