Search API
With the 2024-2025 influenza season underway in the United States and cases of swine flu being reported in people attending county fairs, an innovative vaccine candidate may soon solve these severe health issues.
Emergex Vaccines Holding Limited recently announced that it received patent protection from the United States Patent and Trademark Office (USPTO) for its novel class of influenza vaccines.
This USPTO patent covers Emergex’s vaccine candidate, which comprises immunogenic peptides encoded by a negative sense open reading frame (ORF) from segment 8 of the influenza A genome. This vaccine can potentially provide long-term T-cell immunity against legacy strains of influenza A, seasonal variants, and heterosubtypic changes.
To Emergex’s knowledge, this represents the first known patent for viral peptides derived from antigenomic translation, suggesting that segment 8 of influenza A is ambisense (negative and positive sense ORFs).
In addition, this grants the company exclusive rights to develop a vaccine that incorporates these immune elements, offering a level of immune recognition that existing flu vaccines cannot provide because of composition or method of administration.
Additionally, incorporating avian—and equine species-specific NEG8-derived peptides in a vaccine can potentially expand protection against zoonotic transmissions.
This is an essential feature of a universal flu shot. As influenza viruses change from year to year, influenza vaccines must be updated annually to include the viruses that will most likely circulate in the upcoming season.
Professor Thomas Rademacher, Co-Founder and Chief Executive Officer at Emergex, commented in a press release on August 22, 2024, “Our research into NEG8 has revealed exciting potential for a new approach to influenza vaccines. We believe that a vaccine composition including conserved NEG8-derived MHC class I peptides could protect against past, existing, and emerging human influenza viruses and prevent zoonotic influenza viruses from establishing themselves in the human population and causing a pandemic."
Emergex is set to advance its first-in-class influenza vaccine into the clinic, with Phase I trials anticipated to begin in the first half of 2025.
As of August 25, 2024, various U.S. FDA-approved cell, egg, and nasal flu shots are readily available at health clinics and pharmacies in the U.S.
Over 157 million flu shots were distributed during the 2023-2024 season.
In the U.S., flu vaccines for the 2024-2025 season will be trivalent, and most (91%) will be thimerosal-free or thimerosal-reduced vaccines. About 21% of flu vaccines will be egg-free.

For the first time, messenger RNA (mRNA) immunotherapy will be studied in a phase 1 clinical trial for lung cancer in the UK, where the University College London Hospitals (UCLH) Clinical Research Facility is the lead research site.
As of August 23, 2024, a lung cancer patient at UCLH became the first person to receive a novel cancer vaccine candidate designed to prime the immune system to recognize and fight cancer cells.
Made by BioNTech SE, the investigational mRNA cancer immunotherapy/vaccine for non-small cell lung cancer (NSCLC) known as BNT116 is designed to enhance immune responses against targets primarily expressed by cancer cells.
This process reduces the risk of toxicity to healthy, non-cancerous cells—unlike chemotherapy, which often affects both cancerous and healthy cells.
UCLH consultant medical oncologist Siow Ming Lee, who leads the national study, said in a press release, “Lung cancer remains the leading cause of cancer deaths worldwide, with an estimated 1.8 million deaths in 2020."
“We are now entering this exciting new era of mRNA-based immunotherapy clinical trials to investigate lung cancer treatment, thanks to the foundation laid by the Office for Life Sciences within the Department for Science, Innovation and Technology and the Department for Health and Social Care.
The trial will enroll patients at different stages of NSCLC, from early-stage NSCLC before surgery or radiotherapy (Stages 2 and 3) to late-stage disease (Stage 4) or recurrent cancer.
The trial aims to establish the safety profile and safe dose of BNT116 monotherapy combined with established NSCLC treatments to see if it has a synergistic anti-tumor effect when given with these established chemotherapy or immunotherapy treatments.
Approximately 130 participants will be enrolled in the study across 34 research sites in seven countries, with six UK sites selected. Patients interested in participating in the trial should be directed to their GP or oncologist in the first instance, and their doctor can refer them to the study's trial centers to assess suitability.
BNT116 is also being evaluated in a Phase 2 trial as a first-line treatment for patients with metastatic NSCLC in combination with cemiplimab, a PD-1 inhibitor, and cemiplimab alone.
Bavarian Nordic A/S recently announced it received a new contract of 440,000 doses to supply its MVA-BN® smallpox and mpox vaccine from an undisclosed European country.
The Company confirmed all vaccines under this contract will be delivered in 2024.
Immediate access to these vaccines is essential since the mpox clade 1b outbreak is spreading in Africa.
Paul Chaplin, President & CEO of Bavarian Nordic, said in a press release on August 21, 2024, “Bavarian Nordic can still supply up to 10 million doses of our smallpox and mpox vaccine by the end of next year, with 2 million doses of this capacity available during the remaining part of this year.”
The MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic (JYNNEOS®, IMVANEX®, and IMVAMUNE®) is a non-replicating vaccine approved by several countries, including the United States.

Moderna, Inc. announced that the European Commission (EC) has granted marketing authorization for mRESVIA®, an mRNA respiratory syncytial virus (RSV) vaccine.
RSV is a highly contagious seasonal respiratory virus that causes an exceptionally high burden of disease in infants and older adults.
As of August 23, 2024, this EC authorization is indicated to protect adults aged 60 years and older from lower respiratory tract disease caused by RSV infection.
The marketing authorization is valid in all 27 EU member states, as well as Iceland, Liechtenstein, and Norway.
"The EC's approval of mRESVIA is an important milestone for public health and highlights Moderna's mRNA leadership," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.
In the European Union, RSV is estimated to cause approximately 160,000 hospital admissions in adults each year, with 92% of these admissions occurring in adults aged 65 and over.
In the United States, the RSV season has already begun in Florida and is expected to spread throughout the U.S.
For the 2024-2025 RSV season in the U.S., three vaccines and one monoclonal antibody were approved by the U.S. FDA.
Novavax Inc. today announced, 'We are working productively with the U.S. Food and Drug Administration (FDA) as they complete their review, including providing additional information as needed, and the FDA has committed to moving swiftly on regulatory authorization.'
'We expect to have authorization in time for peak vaccination season.'
Novavax filed for U.S. Emergency Use Authorization of our 2024-2025 formula protein-based COVID-19 vaccine (NVX-CoV2705) in June 2024.
In the United States, Novavax's products have been and will be available after FDA authorization in thousands of locations nationwide, including pharmacies.
For example, Walgreens recently confirmed their pharmacists are available to help patients navigate the latest vaccination guidance, including the timing of vaccinations, given the uneven geographical spreading of the coronavirus.
Additionally, numerous countries have authorized Novavax's COVID-19 vaccines over the past few years.
Novavax wrote on August 22, 2024, 'Our 2024-2025 formula COVID-19 vaccine targets JN.1, the "parent strain” of currently circulating variants and should provide acceptable coverage and cross-reactivity against JN.1 lineage viruses, including KP.2.3, KP.3, KP.3.1.1 and LB.1.1.'
'Upon authorization, Novavax’s vaccine will be the only protein-based option available in the U.S. for individuals aged 12 and older to prevent COVID-19.'
On August 8, 2024, Novavax reported it achieved total revenue of $415 million in the second quarter of 2024 and ended the period with $1.1 billion in Cash.
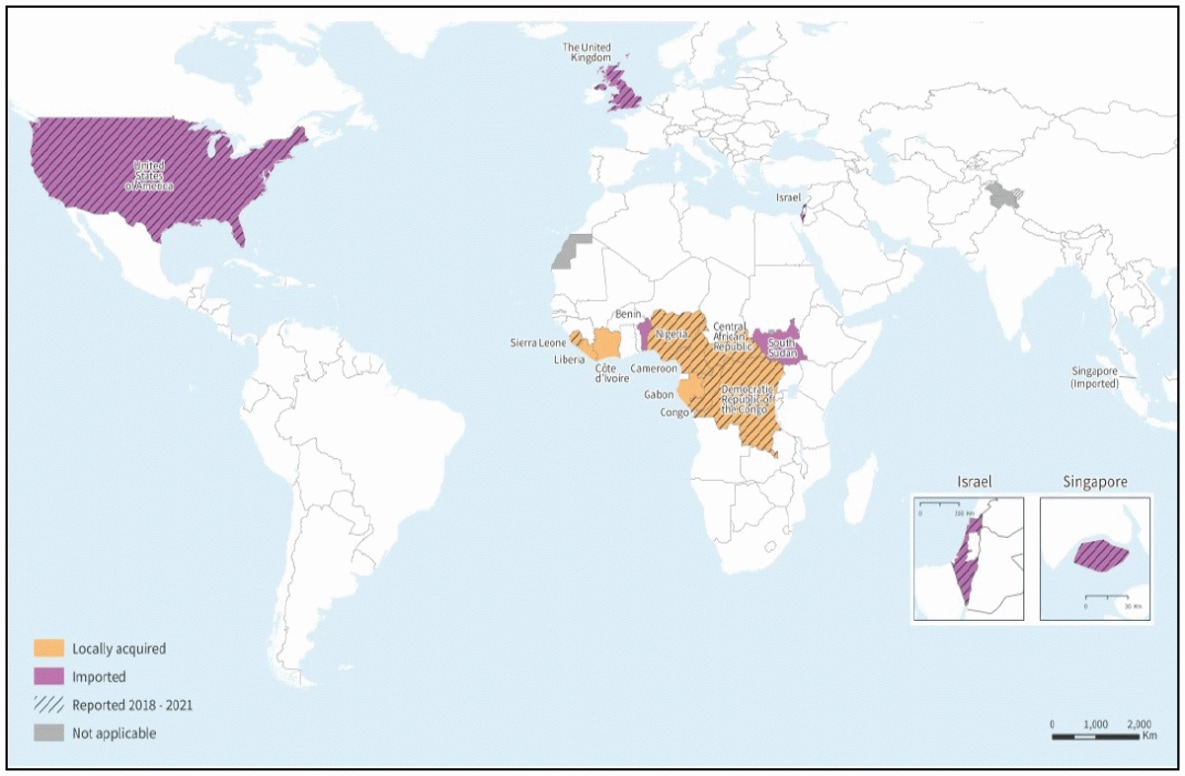
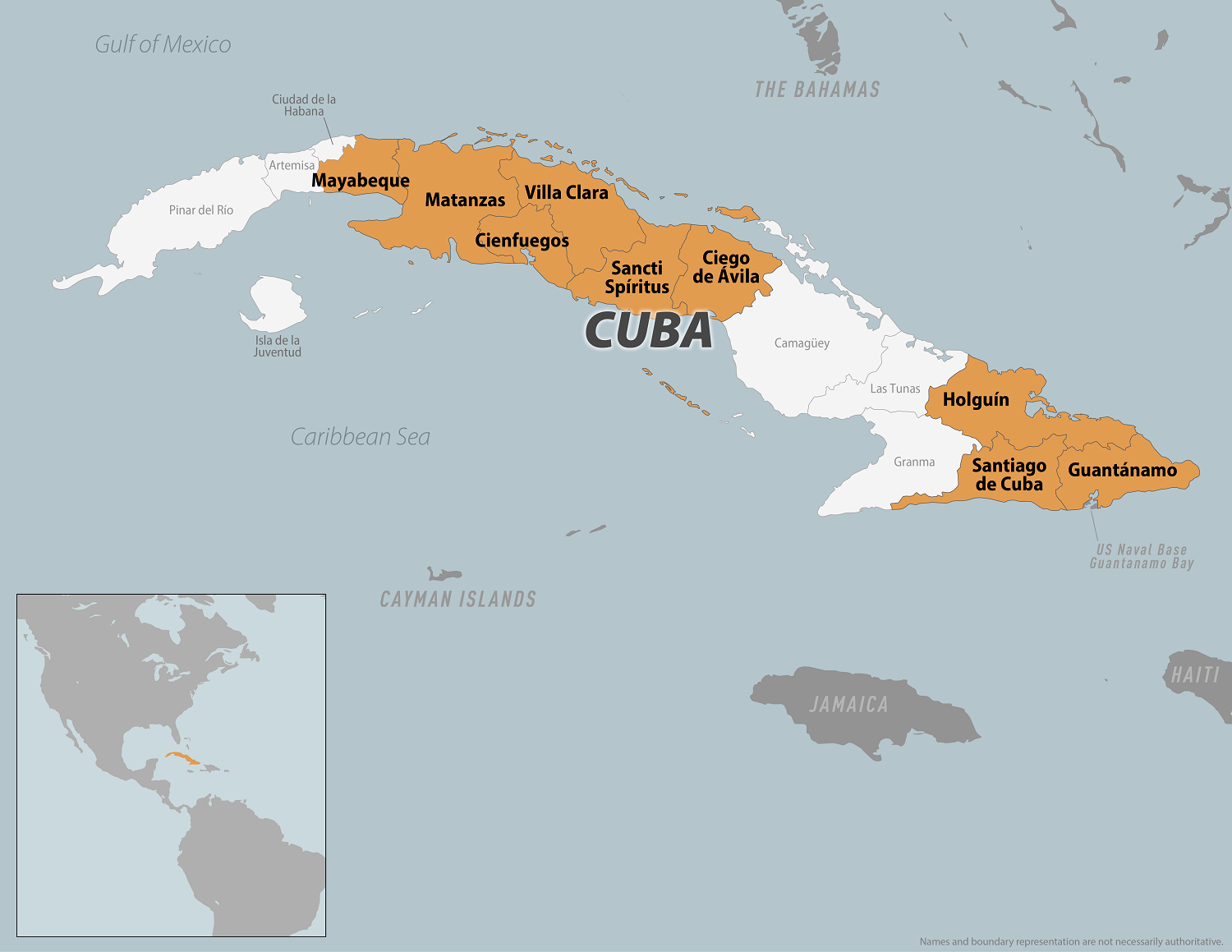
Cuba's Minister of Public Health, José Ángel Portal Miranda, recently announced over 400 Oropouche virus disease cases have been confirmed on the island since late May 2024.
According to Cuba Headlines reporting, Cuba ranks second in the Region of the Americas for the number of infections behind Brazil (7,284).
Infected biting midges and some mosquitoes are spreading the virus.
Currently, there are no efficient vector control measures for the Culicoides paranesis.
As of August 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) says there is no evidence of local transmission of Oropouche virus disease in the United States. However, various states, such as Florida (12), report travel-related cases.
The virus was first detected in 1955 in Trinidad and Tobago near the Oropouche River. Since then, outbreaks of the Oropouche virus have been reported in Bolivia, Brazil, Colombia, Ecuador, French Guiana, Panama, and Peru.
The incubation period for Oropouche virus disease is 3–10 days, says the U.S. CDC. Typically, the disease starts with the abrupt onset of fever (38-40°C), followed by a headache, chills, myalgia, and arthralgia.
People typically recover without long-term sequelae. However, there have been a few deaths reported and vertical transmission of Oropouche virus causing fetal deaths and congenital abnormalities.
The best way to protect themselves from Oropouche is to prevent bites from biting midges and mosquitoes.
According to the CDC's Level 2 Travel Health Advisory, updated on August 15, 2024, travelers to Cuba should prevent bug bites during visits to protect themselves from infection, as there are no vaccines to prevent Oropouche virus disease.
Note - Headlines was edited on Aug, 24, 2024,
The World Health Organization (WHO) announced today that three vaccines are available to prevent mpox in different countries.
Published on August 22, 2024, the WHO's Disease Outbreak News confirmed the MVA-BN® (JYNNEOS®, IMVAMUNE®), LC16-KMB, and OrthopoxVac are available in certain countries. However, OrthopoxVac has not yet been commercialized.
Based on extensive clinical research, the WHO recommends using MVA-BN or LC16 vaccines when the others are not available.
While the ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections, the WHO does not recommend it.
Furthermore, mpox vaccination is recommended by WHO and the U.S. CDC for individuals at high risk of exposure, such as when visiting mpox outbreak areas.