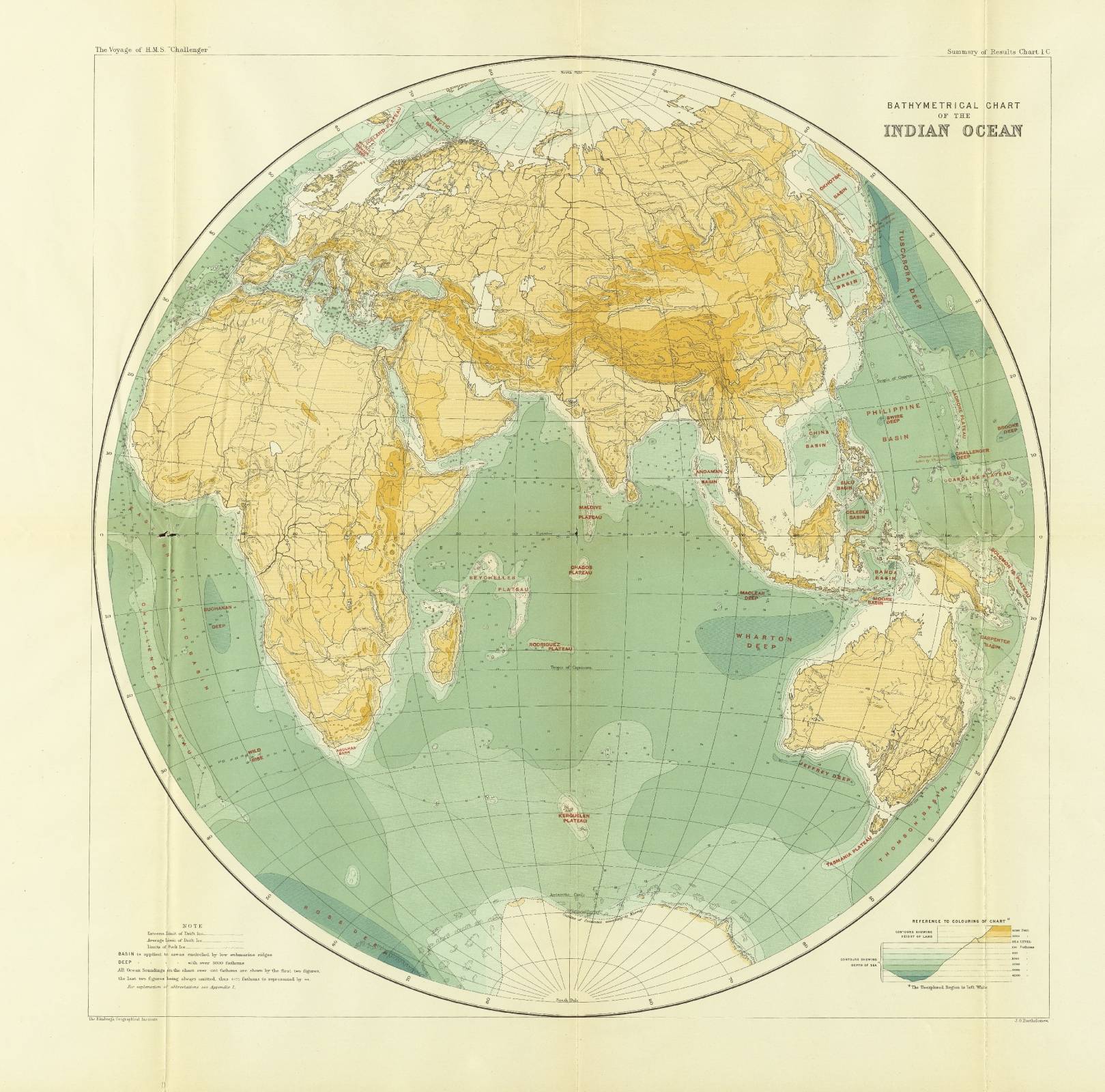
Hajj and Umrah Vaccinations
Hajj Umrah Vaccination Requirements 2025
The Saudi Ministry of Health established vaccine requirements for pilgrims to obtain an Entry Visa for Hajj and to perform Umrah in the Kingdom of Saudi Arabia (KSA). Health regulations and vaccination requirements were updated in 2024. On October 21, 2024, the World Health Organization (WHO) and the KSA announced a new effort to expand the Hajj health smart card initiative that summarizes critical health information, such as immunization status. In 2024, about 250,000 pilgrims from Indonesia, Malaysia, and Oman were issued Hajj health cards as part of the pilot collaboration between WHO and the KSA.
Hajj 1445 vaccinations include the following vaccines:
COVID-19: The Ministry of Health of Saudi Arabia recognizes the World Health Organization's Listed COVID-19 vaccines.
Influenza: The Saudi Ministry of Health recommends vaccinating pilgrims against seasonal flu before arrival in the Kingdom of Saudi Arabia. This is particularly important for those at increased risk of severe influenza, including pregnant women, children under five years of age, the elderly, and individuals with underlying health conditions.
Meningococcal: Adults and children of nine months or older arriving for Umrah, Hajj, or seasonal work must submit a vaccination certificate against meningitis that they have received the (ACYW135) vaccine issued no more than three years and no less than ten days before arrival in Saudi Arabia. In April 2025, 11 cases of invasive meningococcal disease in the KSA were reported to the WHO.
Additional diseases underscore the need for rigorous surveillance and targeted vaccination strategies to mitigate the risk of transmission during the Hajj. Suggested vaccinations include:
Dengue and Zika Viruses: Aircraft, ships, and other means of transportation arriving in KSA from countries affected with the Zika virus and/or Dengue Fever. Valid certificate indicating that disinsection was applied per the methods recommended by WHO. They may be subjected to inspection as a condition of granting free pratique.
Polio: All visitors from polio-endemic countries and re-established transmission countries should receive one oral polio vaccine (OPV) dose, regardless of age and vaccination status. Proof of vaccination at least six weeks before departure is required for visitors from polio-endemic and re-established transmission countries to apply for an entry visa for Saudi Arabia, and travelers will also receive one dose of OPV at border points on arrival in Saudi Arabia. In addition, irrespective of previous immunization history, all visitors under 15 years old arriving in Saudi Arabia will receive one dose of OPV at border points.
Yellow Fever: Following the International Health Regulations 2005, all travelers arriving from countries or areas at risk of yellow fever must present a valid International Certificate of Vaccination or Prophylaxis showing that the person was vaccinated with either FY-Vax or Stamaril vaccine at least ten days before arrival. In the absence of such a certificate, the individual will be placed under strict surveillance.
General Precautions: It is recommended that all pilgrims update their immunizations against vaccine-preventable diseases. These usually include diphtheria, tetanus, pertussis, measles, and mumps. Pilgrims planning travel before or after Hajj or Umrah to malaria-risk areas in Asia, Africa, and Latin America should seek advice.
Vaccinations for Pilgrims Within the Kingdom
Primary healthcare centers located in the Kingdom offer vaccinations.
Travel Vaccinations Saudi Arabia
The U.S. Department of State confirms that those wishing to travel to Saudi Arabia to visit Medina or perform Hajj/Umrah should review the U.S. Centers for Disease Control and Prevention (CDC) vaccine recommendations. If you travel to Saudi Arabia, ensure you are fully vaccinated before travel, says the CDC. The ECDC reported in 2023 that returning travelers from the Hajj should seek medical attention immediately if they experience symptoms suggestive of any infection.