Search API
The U.S. FDA issued a Drug Safety Communication regarding hand sanitizers on June 22, 2021, warning that symptoms such as headache, nausea, and dizziness can occur after applying alcohol-based hand sanitizers to the skin and inhaling the vapors that linger.
The FDA has reviewed case reports and cases from calls to U.S. poison control centers of adverse events after applying alcohol-based hand sanitizers to the skin. While most cases resulted in minor or minimal effects, some cases resulted in treatment by a health care professional.
'Consumers should use hand sanitizer in a well-ventilated area,' says the FDA.
The beta coronavirus that causes COVID-19 is constantly changing through mutation, leading to new variants, including some classified as variants of concern (VOCs), stated the US Centers for Disease Control and Prevention (CDC) on June 25, 2021.
A recent CDC study published on June 11th found that the circulation of SARS-CoV-2 variants in the USA changed rapidly from December 2020 to May 2021, demonstrating how quickly a new variant can emerge.
Currently, several variants are found around the world.
On June 15, 2021, the B.1.617.2 (Delta) variant was classified as a VOC because it spreads from person to person more easily than other variants. Delta has been reported in 77 countries, and in the United Kingdom, it has become the main variant identified in COVID-19 cases.
In the USA, the proportion of Delta variants is predicted to increase to about 20% nationally in late June 2021. Compared with the highest peak in January 2021, the current 7-day average in the USA decreased 95%.
Furthermore, the 7-day average of percent positivity from tests is now 1.7%
If you have questions or concerns about vaccines, please contact your healthcare professional, local health department, or pharmacist or visit the CDC website.
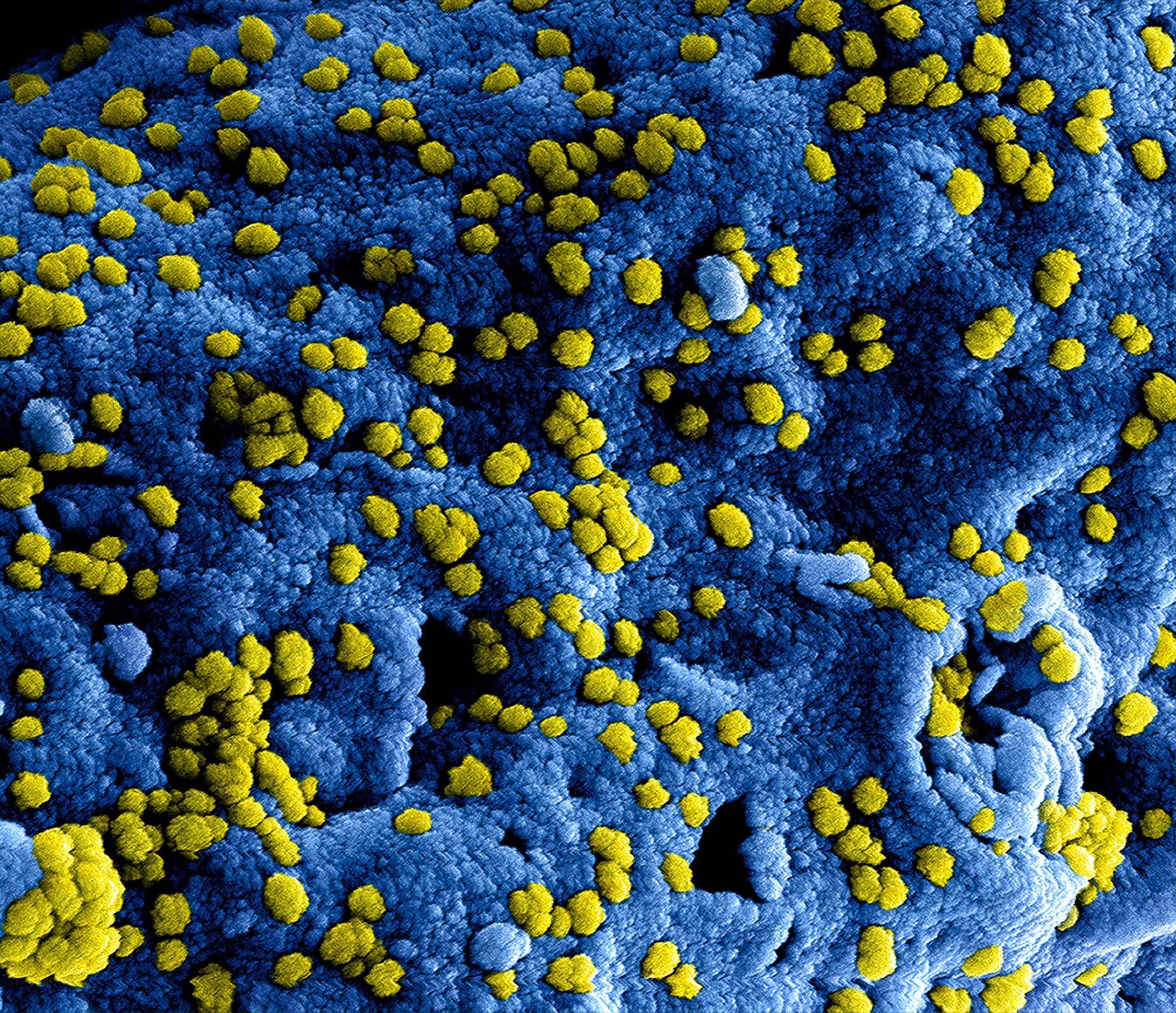
Developing a viable vaccine against the dengue virus has proved difficult for manufacturers because the pathogen is actually four different virus serotypes. Unless a vaccine protects against all four, it can wind up doing more harm than good.
To help vaccine developers overcome this hurdle, the UNC School of Medicine lab of Aravinda de Silva, Ph.D., professor in the UNC Department of Microbiology and Immunology, investigated samples from children enrolled in a dengue vaccine trial to identify the specific kinds of antibody responses that correlate with protection against dengue virus disease.
In doing so, the researchers discovered that a small subpopulation of antibodies binding to unique sites on each serotype is linked to protection.
“Our results suggest that a safe and effective dengue virus vaccine needs to stimulate neutralizing antibodies targeting unique sites on each of the four dengue serotypes. Not merely the neutralizing antibodies against cross-reactive epitopes common to all four dengue types.”
The research was published in the Journal of Clinical Investigation on May 18, 2021, provides important information for vaccine developers to consider when creating a dengue vaccine, which has long eluded scientists.
The U.S. FDA authorized the Dengvaxia vaccine for limited use on May 1, 2019, for people with laboratory-confirmed previous dengue infection and living in endemic areas. And on June 24, 2021, the U.S. CDC's Advisory Committee on Immunization Practices presented updated dengue vaccine considerations.

Researchers at Penn State College of Medicine announced two studies that demonstrate misinformation on social media might affect parents’ willingness to have their children vaccinated against human papillomavirus (HPV).
In the first study, a group of investigators conducted an experimental comparison of vaccine-related tweets.
In the second study, a team of researchers assessed parental support for implementing social media standards to combat misinformation.
These studies demonstrate social media’s important role in disseminating health-related information, according to the researchers. In addition, they noted that messages that evoked emotion and used storytelling were more likely to motivate parents.
Overall, parents supported less restrictive measures such as having information reviewed for accuracy before posting it online (51%) or making anti-vaccine content less common in newsfeeds (32%).
However, parental support was lower for preventing anti-vaccine searches (12%) or disabling accounts that post-anti-vaccine content (16%), stated these researchers on June 10, 2021.

Public Health England (PHE) published influenza surveillance results from the winter period, 2020-2021. The Influenza-like-Illness rate for 2020 to 2021 remained much lower than rates observed in previous seasons. There were no confirmed influenza outbreaks reported in the UK between week #40 2020 and week #14 2021.
The PHE's new report published on June 25, 2021, indicates flu vaccine uptake in England in the 65 years and over cohort, and the 2 and 3-year-old cohorts during 2020-2021 was the highest ever achieved.
Vaccine uptake in the 65 and over cohort was 80.9%, compared to 72.4% in 2019 to 2020.
And uptake in the 2 and 3-year-old cohorts 56.7% compared to 43.8% in 2019 to 2020.
Flu vaccine uptake in the clinically at-risk cohorts is higher than ever previously recorded, at 53.0%, compared to 44.9% in 2019 to 2020.
However, flu vaccine uptake in pregnant women in England in the 2020 to 2021 season remained stable at 43.6%, compared to 43.7% in 2019 to 2020.
Vaccine uptake varied across Wales, Scotland, and Northern Ireland, with increases in uptake seen in most eligible cohorts compared to the previous season, says PHE.
PHE stated, 'Due to the COVID-19 pandemic, data reported from the various influenza surveillance systems must be interpreted with caution and increases seen in some indicators may not accurately reflect influenza activity but rather COVID-19 activity.'

Miami-based Veru Inc. announced that it had enrolled the first patient in its Phase 3 VERACITY clinical trial of sabizabulin, an oral, first-in-class, new chemical entity, androgen receptor transport disruptor, for metastatic castration and androgen receptor targeting agent resistant prostate cancer.
The Phase 3 VERACITY clinical trial is an open-label, randomized, multicenter registration study to evaluate the efficacy and safety of sabizabulin 32mg oral daily dosing versus an alternative androgen receptor targeting agent for the treatment of chemotherapy naïve men with metastatic castration-resistant prostate cancer who have progressed on at least one androgen receptor targeting agent.
The primary endpoint is median radiographic progression-free survival, and key secondary endpoints are overall response rate, duration of objective response, overall survival, time to chemotherapy, and pain progression. The study is expected to enroll 245 patients and will be conducted in over 45 clinical sites across the USA.
Veru Inc. is an oncology biopharmaceutical company focusing on developing novel medicines for the management of prostate cancer and breast cancer.

During the Centers for Disease Control and Prevention (CDC) vaccine meeting on June 25, 2021, Grace Lee, M.D., MPH, led the Herpes Zoster Work Group overview presentation; Zoster Vaccines Session: Burden of Herpes Zoster in Immunocompromised Adults.
The ACIP committee is debating the policy question: “Should vaccination with RZV be recommended for immunocompromised adults 19 years of age and older?”
Robyn Widenmaier, GSK's Global Medical Portfolio Lead, Zoster Vaccine, will follow that session and lead a clinical discussion regarding the Use of Recombinant Zoster Vaccine in Immunocompromised Populations.
GSK's Shingrix is an adjuvanted recombinant zoster vaccine, consisting of the varicella-zoster virus glycoprotein E antigen and the AS01B adjuvant system, a proprietary adjuvant containing QS-21 and MPL with liposomes. This vaccine boosts the human body’s protection against shingles.
On October 20, 2017, the U.S. FDA issued Authorization for the Shingrix vaccine.

The US Centers for Disease Control and Prevention (CDC) vaccine committee will conduct the third day of its regularly scheduled meeting today. Dr. José R. Romero, Arkansas's Secretary of Health, currently leads the Advisory Committee on Immunization Practices (ACIP) committee discussions.
The ACIP's updated agenda for June 25, 2021, focuses on Pneumococcal and Zoster vaccines.
Grace Lee, M.D., MPH, leads the Zoster vaccine discussion, and Katherine A. Poehling, M.D., MPH, will lead the pneumococcal presentation.
Today's ACIP meeting will be held digitally without requiring registration to attend. Any member of the public can submit a written public comment to ACIP. For June 25, 2021, the Docket No. CDC–2021–0034 is being used at the Federal eRulemaking Portal.
The ACIP committee provides advice and guidance to the Director of the CDC, Rochelle P. Walensky, M.D., MPH, on the use of vaccines and related agents for the control of vaccine-preventable diseases. The CDC Director reviews these recommendations and, if adopted, are published as official recommendations in the CDC's Morbidity and Mortality Weekly Report.
As of June 25, 2021, the CDC has authorized three COVID-19 vaccines for emergency use. The U.S. FDA has not approved these experimental vaccines.

Advisory Committee on Immunization Practices (ACIP) will conduct the second day of its vaccine review meeting. Dr. José R. Romero, Arkansas's Secretary of Health, leads the ACIP committee discussions.
The ACIP's updated agenda for June 24, 2021, focuses on three diseases: Dengue, Influenza, and Rabies.
Today's ACIP meeting will be held digitally without requiring registration to attend. Any member of the public can submit a written public comment to ACIP. For the June 23-25 ACIP Meeting, the Docket No. CDC–2021–0034 is being used at the Federal eRulemaking Portal.
The ACIP provides advice and guidance to the Director of the U.S. CDC, Rochelle P. Walensky, M.D., MPH, on the use of vaccines and related agents for the control of vaccine-preventable diseases. The CDC Director reviews recommendations made by the ACIP and, if adopted, are published as official recommendations in the CDC's Morbidity and Mortality Weekly Report.

