Search API
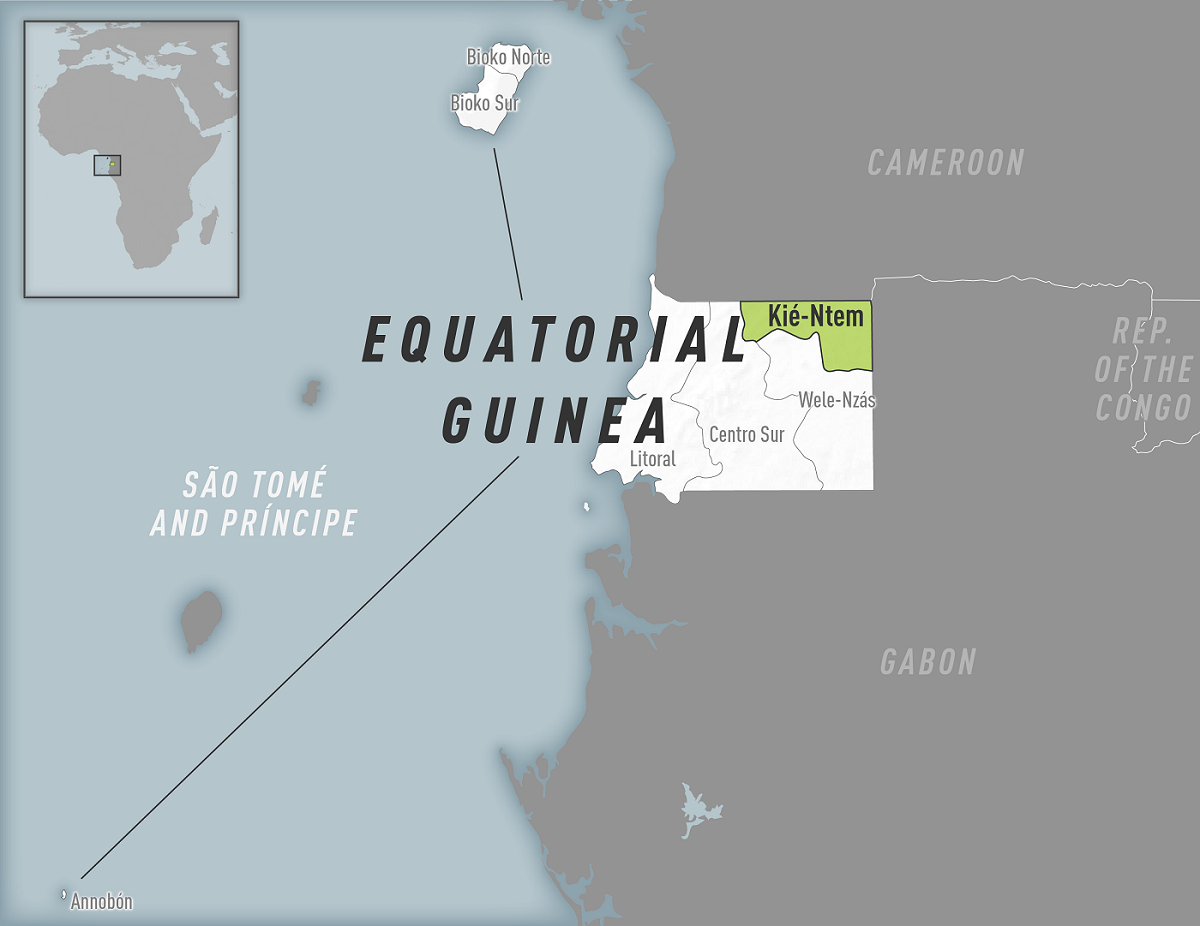
The U.S. Centers for Disease Control and Prevention (CDC) today announced Equatorial Guinea declared an outbreak of Marburg virus disease (MVD) after confirming cases in the Kie Ntem Province.
In response, Equatorial Guinea declared an outbreak of MVD on February 13, 2023.
The CDC stated on February 16, 2023, that MVD is a rare and deadly disease that has sometimes caused outbreaks in several African countries.
Travelers to MVD endemic areas should separate themselves from others and seek medical care immediately if they develop fever, chills, muscle pain, rash, sore throat, diarrhea, weakness, vomiting, stomach pain, or unexplained bleeding or bruising during or after travel (up to 21 days).
And, call ahead before going to a healthcare facility and tell your doctor that you've been to an area reporting MVD cases, says the CDC.
Initially detected in 1967 in Germany, MVD is spread by contact with the blood or body fluids of a person infected with the Marburg virus.
As of February 17, 2023, Angola, DR Congo, Equatorial Guinea, Cameroon, Germany, Ghana, Guinea, Kenya, Serbia, South Africa, and Uganda have previously confirmed MVD cases.
While there are no U.S. FDA-approved Marburg preventive vaccines, several candidates are conducting clinical studies.
NPR reported today Dr. Leana Wen, an emergency physician and professor at the Milken School of Public Health at George Washington University, says there's one more urgent reason a vaccine must be prioritized.
"Healthcare workers are at particular risk, and in Equatorial Guinea and surrounding countries, illness and death of the relatively few doctors and nurses they have would have a tremendous lasting impact on health in their region."
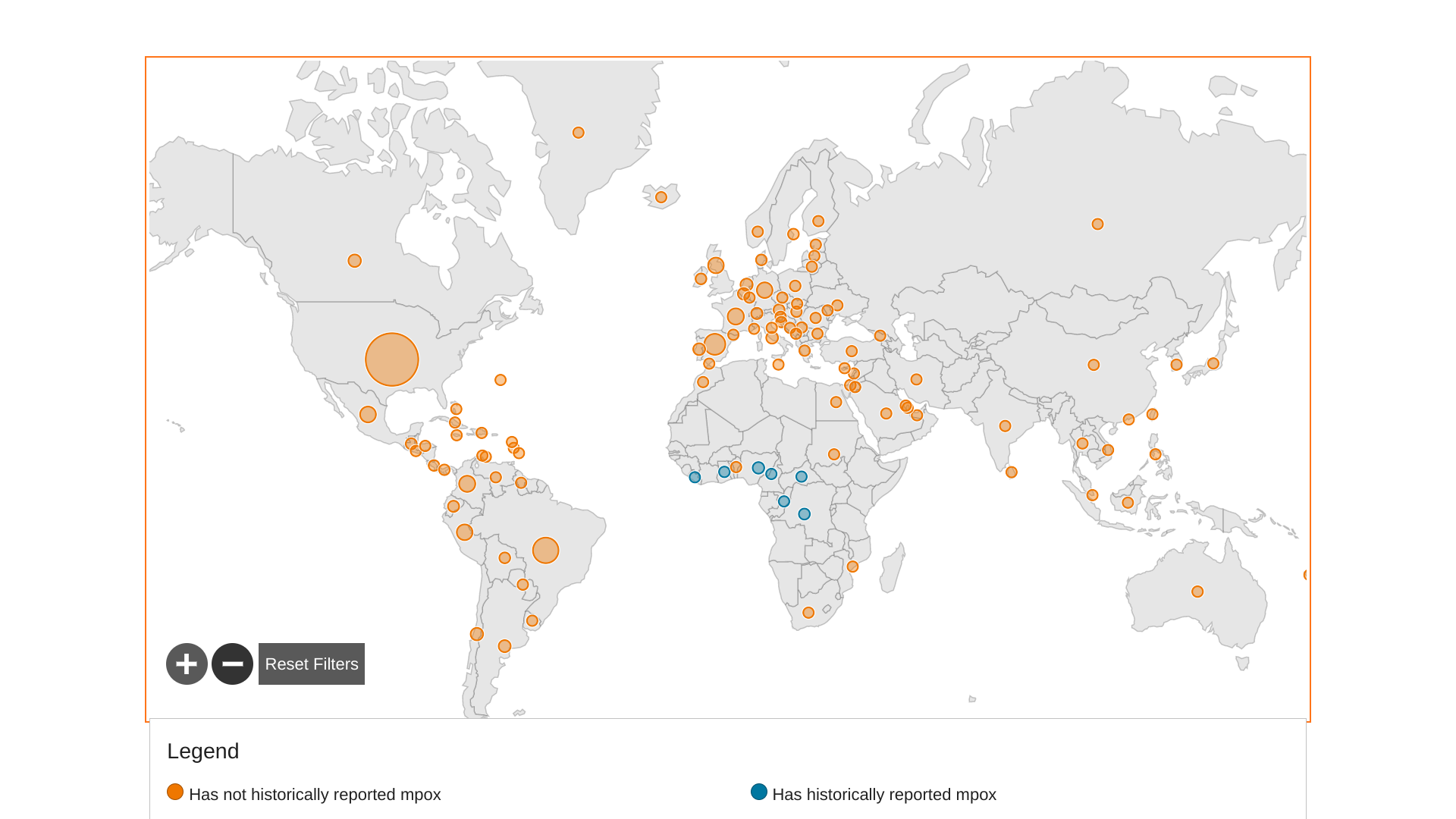
The acting director of the Africa Centers for Disease Control and Prevention (ACDC), Ahmed Ogwell, stated he hopes Mpox vaccines will finally arrive on the continent "in another two weeks, tops" after months of seeking doses.
The AP reported on February 16, 2023, the Mpox vaccines will go first to countries with acute need and the most significant overall burden, such as Congo and Nigeria. Nigeria was listed in the U.S. CDC's Watch - Level 1, Practice Usual Precautions notice issued in November 2021.
People become infected with the mpox virus through contact with the bodily fluids of infected animals or humans.
Mpox occurs throughout Central and West Africa, often near tropical rainforests. The first human case of Mpox was identified in the Democratic Republic of Congo in 1970.
In November 2022, the government of the Republic of Korea, through the Korean Disease Control and Prevention Agency, announced it would donate the first batch of the Mpox vaccine to Africa through the ACDC.
In the U.S., over 1.1 million doses of the Bavarian Nordic JYNNEOS® (MVA-BN) vaccine have been distributed since May 2022.
Since May 5, 2022, about 110 countries have reported 85,800 Mpox patients and 88 related fatalities.
The countries with the highest cumulative notification rates in Europe are Spain, Portugal, and Luxembourg. Additional Mpox outbreak news is posted at MpoxToday.
While the World Health Organization has maintained its multi-country outbreak notice, the U.S. recently discontinued its Mpox alert.
The continual spreading of the Highly Pathogenic Avian Influenza (HAPI) H5N1 influenza A virus across the globe has prompted many questions without clear answers.
These questions accelerated recently when seven people, including various mammals, became infected over the past year.
Amesh Adalja, M.D. with MedPageToday, published an opinion article on February 16, 2023, focused on whether an influenza pandemic caused by this version of HAP is imminent.
'This is an important question that must be asked of all novel influenza viruses. However, the answers regarding influenza are not that simple and require placing this strain into a larger context of avian influenza more generally,' wrote Dr. Adalja. The full unedited article is posted at this link.
Avian influenza A (bird flu) viruses are common and widespread in birds.
Should a human-to-human HAPI outbreak occur, the U.S. Food and Drug Administration has already approved one type of 'bird flu vaccine' that could be effective.
While most people have already received their annual flu shot, this type of vaccine would not be very effective against HAPI viruses.
The World Health Organization (WHO) Influenza Update N° 438 recently reported influenza activity decreased globally.
In the countries of North America, most indicators of influenza activity fell to levels similar to or below levels typically observed this time of year.
As of February 9, 2023, Influenza A(H3N2) was the predominant virus detected in the U.S., whereas A(H1N1)pdm09 and A(H3N2) were co-circulated in Canada in the most recent reporting week.
In Europe, overall influenza activity continued to decrease, but influenza positivity from sentinel sites remained above the epidemic threshold at the regional level.
Separately, a leading mRNA-based flu shot candidate for adults announced encouraging interim results from its pivotal Phase 3 safety and immunogenicity trial of mRNA-1010 (P301).
As of February 4, 2023, the U.S. CDC confirmed about 172.76 million influenza vaccines had been distributed for the 2022-2023 flu season in the U.S.

Moderna Inc. today announced one of five influenza vaccine candidates in its portfolio published interim results from its pivotal Phase 3 safety and immunogenicity trial.
mRNA-1010 (P301) is an mRNA-based seasonal influenza (flu) vaccine candidate for adults.
mRNA-1010 encodes for hemagglutinin (HA) glycoproteins of the four influenza strains recommended by the World Health Organization (WHO) to prevent influenza.
Interim results indicate that mRNA-1010 achieved superiority on seroconversion rates for A/H3N2 and A/H1N1 and superiority on geometric mean titer ratios for A/H3N2 and non-inferiority on geometric mean titer ratios for A/H1N1.
Non-inferiority was not met for either endpoint for the influenza B/Victoria- and B/Yamagata-lineage strains.
"Today's results represent an important step forward in developing mRNA-based influenza vaccines to address the substantial burden of disease caused by influenza."
"We are encouraged by the safety and tolerability profile and the strong immunogenicity results against Influenza A viruses which cause the overwhelming majority of flu-related disease in older adults."
"We now look forward to the efficacy results from the ongoing pivotal Phase 3 efficacy study being conducted in parallel," said Stephen Hoge, M.D., Moderna's President, in a press release on February 16, 2023.
"While we did not achieve non-inferiority for the Influenza B strains, which are more frequent in younger populations, we have already updated the vaccine that we believe could improve immune responses against Influenza B and will seek to quickly confirm those improvements in an upcoming clinical study thanks to the agility of our mRNA platform."
The first per-protocol interim analysis of efficacy is now expected to be reviewed by an independent Data and Safety Monitoring Board (DSMB) before the end of the first quarter of 2023.
Based on these results, the DSMB will notify the Company whether the primary efficacy endpoint has been met or whether the study should continue accruing further cases toward the final analysis.
Moderna is advancing a portfolio of five influenza vaccine candidates that include additional HA antigens for broader coverage of circulating influenza A strains (mRNA-1011 and mRNA-1012) and candidates that incorporate both HA and neuraminidase (NA) antigens to target multiple proteins involved in the influenza virus lifecycle to reduce the potential of viral antigenic escape (mRNA-1020 and mRNA-1030).
Moderna is also developing combination vaccine candidates, including vaccine candidates against influenza and SARS-CoV-2, influenza and RSV, and influenza, SARS-CoV-2, and RSV.
The goal of Moderna's combination vaccine candidates is to protect against multiple respiratory pathogens in a single vaccine.
Moderna's unedited press release is available at this link.
Other flu shot news is posted at PrecisionVaccinations.

ImmunityBio, Inc. today announced Dr. Karim Chamie, Associate Professor of Urology at UCLA, will be presenting “Quality of life in QUILT 3.032 study: Patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) receiving IL-15R⍺Fc superagonist N-803 plus BCG” at the ASCO Genitourinary Cancers Symposium conference on February 16-18, 2023.
N-803 (Anktiva™), ImmunityBio’s lead cytokine fusion protein, is a novel IL-15 superagonist complex and has received Breakthrough Therapy and Fast Track Designations from the U.S. Food and Drug Administration (FDA) for BCG-unresponsive CIS NMIBC.
N-803 is currently under review by the FDA for this indication with a Prescription Drug User Fee Act target date of May 23, 2023.
Previously, on November 10, 2022, the peer-review journal NEJM Evidence published results from the QUILT 3.032 trial studying N-803 plus BCG in adults with NMIBC CIS with or without Ta/T1 papillary disease.
The published results demonstrate that in patients with BCG-unresponsive NMIBC CIS and papillary disease, BCG plus N-803 (referred to as NAI) CRs were achieved with the persistence of effect with a 90% probability of avoiding cystectomies in responders, a life-changing procedure of removing the bladder, and 100% bladder cancer-specific survival at 24 months.
“The peer review and publication of data in NEJM Evidence highlights the significance of the positive results of the QUILT 3.032 trial in patients with BCG-unresponsive NMIBC,” commented Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on November 10, 222.
“These data further our understanding of N-803’s unique role in potentially boosting the proliferation of natural killer and T cells while synergistically enhancing BCG efficacy.”
ImmunityBio is a vertically-integrated, clinical-stage biotechnology company developing next-generation therapies and vaccines that bolster the natural immune system to defeat cancers and infectious diseases.

Recent American Cancer Society (ACS) statistics show a concerning increase in prostate cancer cases, particularly among Black men, wrote Abe Rosenberg with the City of Hope.
On February 15, 2023, the new data indicated cancer mortality rates have decreased by 33% in the last three decades.
After decades of decline, prostate cancer rates increased by 3% annually from 2014 to 2019.
And there is a significant increase among men presenting with later-stage prostate cancer.
Furthermore, according to the ACS report, Black men have a 70% higher prostate cancer incidence rate than white men, and they are two to four times more likely to die of prostate cancer than any other ethnic group.
“People got confused,” commented urologic surgeon Diana Londoño, M.D., assistant clinical professor in the Division of Urology and Urologic Oncology, Department of Surgery, who sees patients at City of Hope’s Glendora clinical network location.
Dr. Londono emphasized the importance of regular screenings, especially postpandemic, as people resume seeing their doctors regularly.
To reach out to underserved communities where cancer rates are high, ACS unveiled a new initiative called IMPACT (Improve Mortality From Prostate Cancer Together), aimed at reducing prostate cancer disparities in Black men and deaths from prostate cancer for all men by 2035.
The Center for Community Outreach and Engagement runs several programs aimed at the same result.
The City of Hope is dedicated to making a difference in the lives of people with cancer, diabetes, and other life-threatening illnesses.

The Public Health Agency of Canada (PHAC) today issued a statement offering an update on the ongoing response to the Mpox outbreak.
On February 9, 2023, the World Health Organization (WHO) Director-General declared that Mpox remained a Public Health Emergency of International Concern.
This initial declaration was issued on July 23, 2022.
On February 14, 2023, the WHO noted a sustained decline in Mpox cases globally, with the majority of cases being reported from the Regions of the Americas, with 200-250 cases per week,
And about 4% of Mpox cases occurred in women.
Since the beginning of the Mpox outbreak, the Government of Canada has taken action to protect the health and safety of Canadians.
The ongoing management of Mpox relies on continued vigilance, re-emergence of cases, various public health measures, and vaccination.
Mpox vaccines will continue to be available in Canadian provinces and territories for those at higher risk, including second doses of Imvamune®.
Bavarian Nordic's MVA-BN vaccine is approved by the U.S. Food and Drug Administration for smallpox and Mpox under the JYNNEOS® brand.
Since May 2022, the Government of Canada has deployed over 145,000 doses of vaccine.
Mpox information can be found on the Government of Canada's Mpox: For health professionals website. Other outbreak news is posted at Mpox Today.