Search API
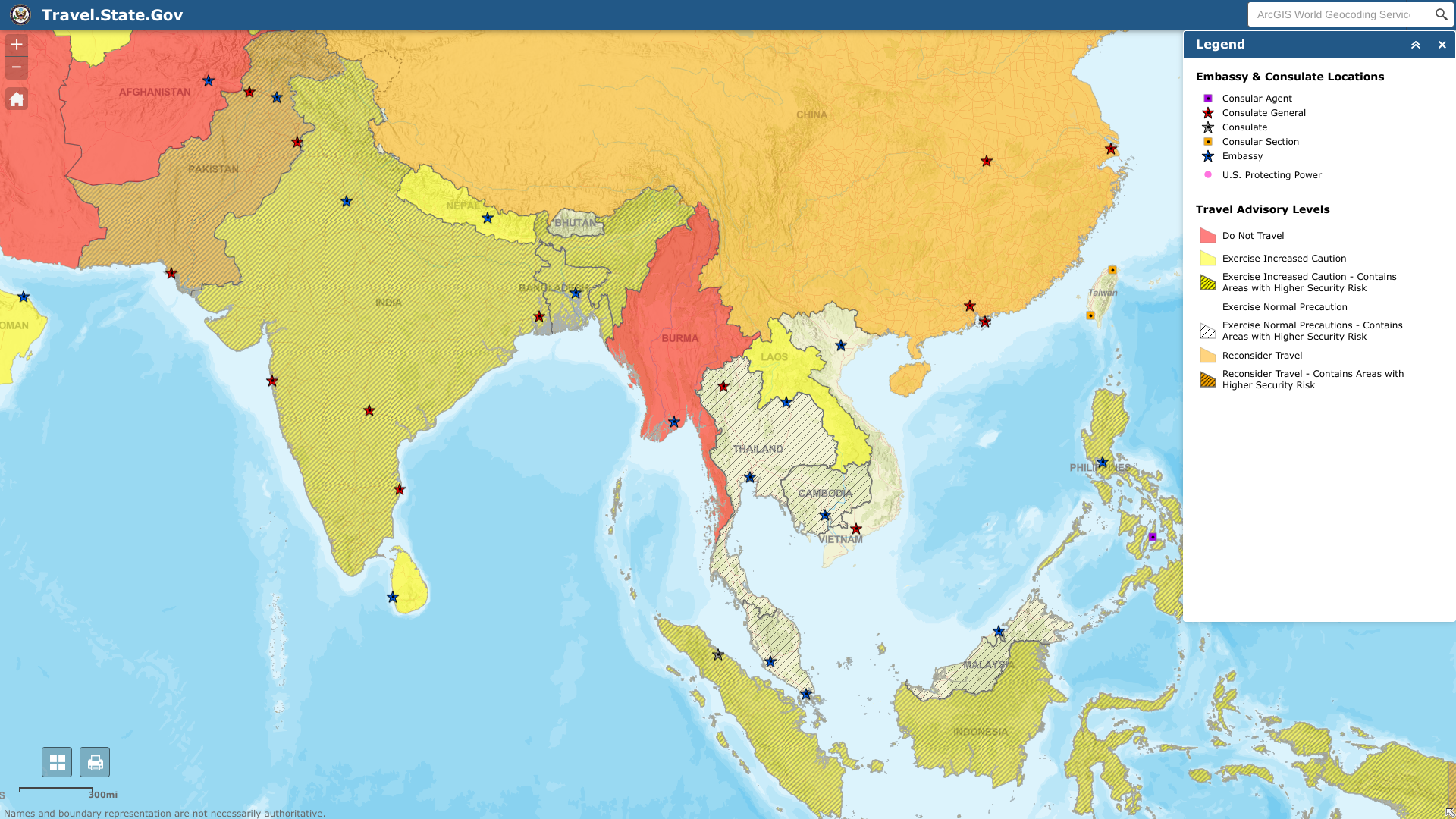
The U.S. Department of State recently reissued its high-level travel advisory for the Republic of the Union of Myanmar, known as Burma.
On February 27, 2023, the State Department published a Level 4: Do Not Travel notice for Burma regarding civil unrest and inadequate access to healthcare resources.
Additionally, the U.S. government has determined that the risk of wrongful detention of U.S. nationals exists, and it has limited ability to provide emergency services in Burma.
If Americans need assistance in the country, the U.S. Embassy Rangoon is at 110 University Ave, Kamayut Township, Rangoon, Burma.
Statista recently reported that as of 2018, the number of international tourist arrivals in Myanmar amounted to approximately 4.2 million arrivals, most via land routes.
From a health perspective, the U.S. CDC included Burma in a Dengue outbreak travel alert issued in 2022. And the importation of medical supplies, including medicine, is not consistent and may not be available.
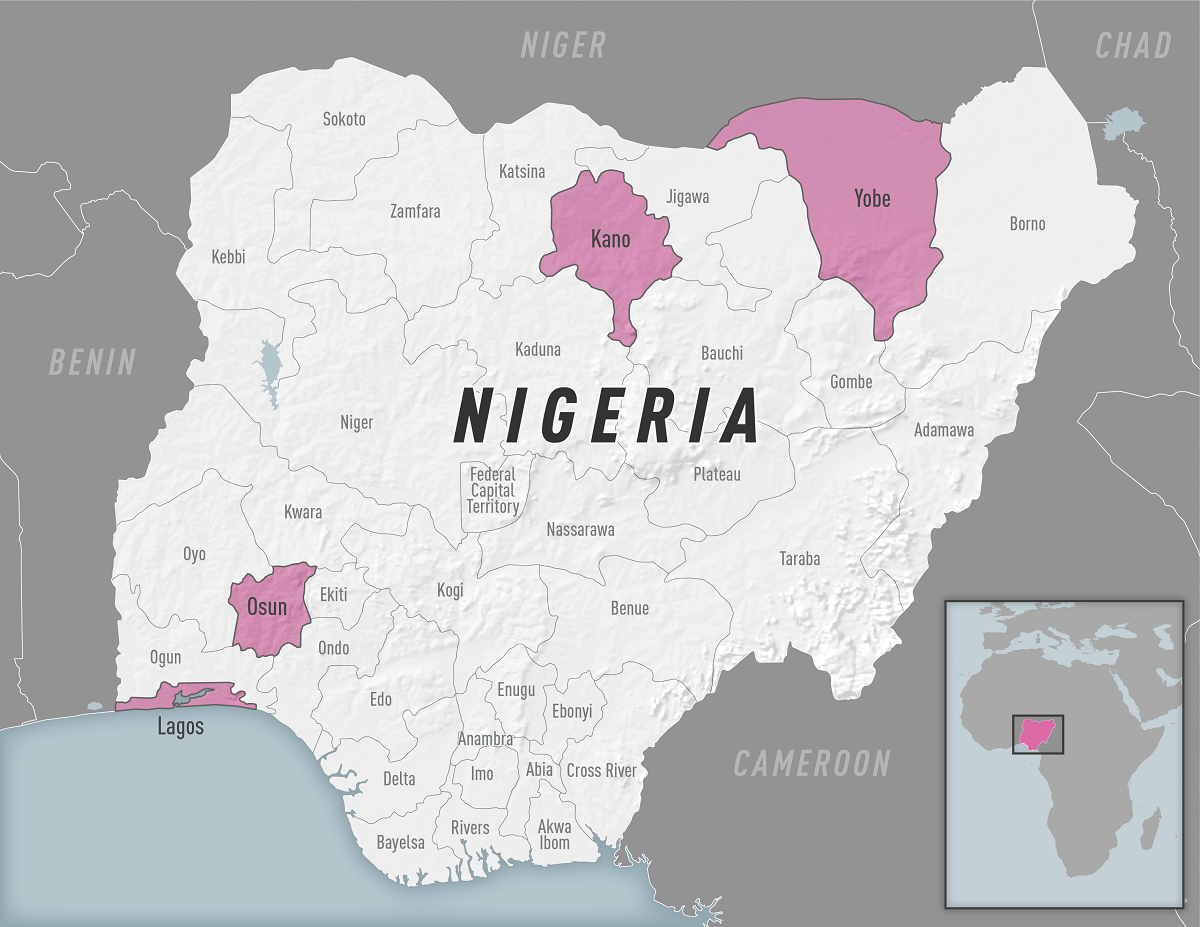
The U.S. Centers for Disease Control and Prevention (CDC) recently added another travel advisory focused on disease outbreaks in the Federal Republic of Nigeria.
On February 24, 2023, the CDC issued its latest advisory focused on an outbreak of diphtheria in several states in Nigeria.
This Alert - Level 2, Practice Enhanced Precautions, says vaccination against diphtheria is essential to protect people against disease.
If traveling to an affected area in Nigeria, you should be updated with diphtheria vaccinations. Furthermore, discuss the need for a booster dose with your healthcare professional before traveling.
CDC recommends vaccinating everyone two months and older to protect against diphtheria.
Diphtheria is a serious infection caused by strains of Corynebacterium diphtheriae bacteria that make a toxin that can cause people to get very sick.
The diphtheria bacteria spread from person to person through respiratory droplets, like from coughing or sneezing; people can also get sick from touching open sores or ulcers of people ill with diphtheria.
Without treatment, up to half of patients can die from the disease, says the CDC.
The CDC previously issued travel advisories for Nigeria's vaccine-preventable diseases, such as yellow fever, polio, measles, and Mpox outbreaks.
These travel vaccines are offered in the U.S. at certified clinics and pharmacies.
The Centre for Health Protection (CHP) of the Department of Health today announced it is closely monitoring a human case of avian influenza A(H5N6) in the Mainland.
As of March 1, 2023, the 'bird-flu' case involves a 49-year-old man living in Qingyuan, Guangdong, who had contact with live domestic poultry before the onset of symptoms on December 17, 2022. He was admitted for treatment on December 21, 2022, and was in serious condition.
"All novel influenza A infections, including H5N6, are notifiable infectious diseases in Hong Kong," a spokesman for the CHP said in a press release.
Travelers to the Mainland or other affected areas must avoid visiting wet markets, live poultry markets, or farms. And they should be alert to the presence of backyard poultry when visiting relatives and friends.
Furthermore, travelers returning from affected areas should consult a doctor promptly if symptoms develop and inform the doctor of their travel history for prompt diagnosis and treatment of potential diseases.
The CHP reported from 2014 to date, 83 human cases of avian influenza A(H5N6) have been reported by Mainland health authorities.
While local surveillance, prevention, and control measures are in place, the CHP will remain vigilant and work closely with the World Health Organization and relevant health authorities to monitor the latest developments.
Bird Flu (Avian influenza) is a disease caused by influenza type A viruses that occur naturally among birds and can infect humans. And Highly Pathogenic Avian Influenza (HAPI) viruses carrying the H1N1, H2N2, and H3N2 combinations were responsible for the outbreaks such as the Spanish flu of 1918, the Asian flu in 1957, and the Hong Kong flu in 1968,
HAPI virus subtypes (H5, H7, H9) A(H5N1) emerged in southern China in 1997.
Bird flu preventive vaccines have limited availability in the U.S. as of March 2023.

Pfizer Inc. announced today that the U.S. Food and Drug Administration's (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted during its 179th meeting that available data is adequate to support the safety and effectiveness of its respiratory syncytial virus (RSV) bivalent vaccine candidate ABRYSVO™ (PF-06928316 or RSVpreF).
The Committee voted 7 to 4 on safety and 7 to 4 on effectiveness.
The vaccine candidate is under FDA review to prevent acute respiratory disease and lower respiratory tract disease caused by RSV in adults 60 and older.
The FDA's decision on whether or not to approve RSVpreF is expected by the Prescription Drug User Fee Act (PDUFA) goal date in May 2023.
Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, commented in a related press release on February 28, 2023, "We are encouraged by the outcome of today's VRBPAC meeting as it is a testament to the strength of our science and dedication to bringing this important vaccine candidate to the market."
"We look forward to working with the FDA as it completes the review of our application."
Pfizer is currently the only company pursuing regulatory applications for an RSV investigational vaccine candidate for both an indication to help protect older adults, as well as a maternal indication to help protect infants through maternal immunization.
Regarding the maternal indication, earlier this month, Pfizer announced that the FDA had granted priority review to a biologics license application for RSVpreF for the prevention of lower respiratory tract and severe lower respiratory tract disease caused by RSV in infants from birth up to six months of age by active immunization of pregnant women.
The FDA established a PDUFA action date in August 2023.
The role of the VRBPAC is to provide recommendations to the FDA; however, these recommendations are not binding.
RSV is a contagious virus and a common cause of respiratory illness.
The virus can affect the lungs and breathing passages of an infected individual. As a result, it can potentially cause severe illness in young infants, older adults, and individuals with certain chronic medical conditions.

The Equatorial Guinea Health Ministry tweeted today confirming two additional Marburg Virus Disease (MVD) deaths, which increases the outbreak total to 11 fatalities.
An official statement issued on February 27, 2023, also stated there are 4 other suspected MVD cases. Overall, 48 contacts have been identified.
Equatorial Guinea confirmed its first-ever outbreak of MVD in the Kie Ntem Province on February 13, 2023.
The World Health Organization recently assessed the risk posed by the MVD outbreak as high at the national level, moderate at the regional level, and low at the global level.
For example, Spain reported an MVD case in a returning traveler last week.
Given these continued outbreak concerns, the U.S. CDC previously issued a Watch - Level 1, Practice Usual Precautions, travel alert.
Marburg is a severe human disease caused by the Marburgvirus and can potentially cause epidemics with significant case fatality rates of 50%.
It is spread by contact with the blood or body fluids of a person infected with Marburgvirus.
It is also spread by contact with contaminated objects or animals, such as bats and nonhuman primates infected with Marburgvirus.
As of February 28, 2023, the African CDC, the U.S. Food and Drug Administration, and the European Medicines Agency have not approved any Marburg vaccine candidate.

During the initial months of 2023, millions of people were frustrated by the lack of herpes simplex virus (HSV) vaccine development news.
As of February 28, 2023, the U.S. Food and Drug Administration has not authorized any HSV vaccine.
While herpes-candidate vaccines have been conducting clinical research for years, recent messenger RNA (mRNA) technology announcements raised hopes in 2022
These mRNA herpes vaccines are being produced by industry leaders Moderna Inc. and BioNTech SE.
Moderna's herpes simplex virus (HSV) vaccine candidate (mRNA-1608) is an mRNA vaccine targeted against HSV-2 disease, focused on inducing a strong antibody response with neutralizing functionality combined with cell-mediated immunity.
However, mRNA-1608 has yet to launch a human-based phase 1 clinical study.
Based on BioNTech's mRNA platform, the BNT163 candidate vaccine encodes three HSV-2 glycoproteins to help prevent HSV cellular entry and spread and counteract the immunosuppressive properties of HSVs.
BNT163's phase 1 clinical study includes 108 participants and was recently updated on January 5, 2023, and has an Estimated Study Completion Date of June 2025.
Herpes is a sexually transmitted disease (STD), says the FDA. There are two types of HSV that can cause genital herpes: HSV-1 and HSV-2.
Most cases of recurrent genital herpes are caused by HSV-2, and 11.9% of persons aged 14–49 years are estimated to be infected in the U.S.
However, an increasing proportion of anogenital herpetic infections have been attributed to HSV-1, which is prominent among young women.
To increase transparency, the U.S. National Instuties of Health and its partners launched STI Watch, a portal containing updated information on vaccine development status.
Other herpes vaccine news is posted at PrecisionVaccinations.com/Herpes.

Dynavax Technologies Corporation today announced that the United Kingdom's Medicines and Healthcare products Regulatory Agency had granted Marketing Authorization in Great Britain for HEPLISAV B® for the active immunization against hepatitis B virus infection (HBV) caused by all known subtypes of hepatitis B virus in adults.
"Hepatitis B is a highly infectious and potentially deadly virus with increasing infection rates, and over 250 million people infected worldwide. Thankfully, it can be prevented with effective vaccination," commented Ryan Spencer, Chief Executive Officer of Dynavax, in a press release on February 28, 2023.
HEPLISAV-B combines hepatitis B surface antigen with Dynavax's proprietary Toll-like Receptor 9 agonist adjuvant CpG 1018 to enhance the immune response.
HEPLISAV-B is indicated for preventing infection caused by all known subtypes of HBV in adults aged 18 years and older in the U.S.

Anixa Biosciences, Inc. today announced that the U.S. Patent and Trademark Office had issued a Notice of Allowance broadening the protection of Anixa's novel breast cancer vaccine technology.
This technology was invented by the late Dr. Vincent Tuohy and developed at Cleveland Clinic, and Anixa is the exclusive worldwide licensee.
Anixa's breast cancer vaccine candidate, currently in Phase 1 clinical trials, takes advantage of endogenously produced proteins that have a function at certain times in life but then become "retired" and disappear from the body.
One such protein is a breast-specific lactation protein, α-lactalbumin, which is no longer found post-lactation in normal, aging tissues but is present in most triple-negative breast cancers.
Activating the immune system against this "retired" protein provides preemptive immune protection against emerging breast tumors that express α-lactalbumin.
The vaccine candidate also contains an adjuvant that activates an innate immune response, which allows the immune system to mount a response against emerging tumors to prevent them from growing.
Dr. Amit Kumar, Chairman and CEO of Anixa, commented in a press release on February 27, 2023, "This breast cancer vaccine has the potential to prevent Triple Negative Breast Cancer, the deadliest form of breast cancer, and perhaps other forms of breast cancer that express alpha-lactalbumin."
"In addition, with our partners at Cleveland Clinic, we .... plan to present data from the trial at the annual American Association for Cancer Research meeting in April (2023)."
One in eight women in the U.S. will be diagnosed with invasive breast cancer at some point in their lives. Approximately 10-15% of those diagnoses are Triple-Negative Breast Cancer.

The World Health Organization (WHO) recently published its recommended composition of influenza virus trivalent and quadrivalent vaccines for use in the 2023-2024 southern hemisphere influenza season.
The recommendation, published on February 24, 2023, is based on data generated by the WHO Global Influenza Surveillance and Response System.
This periodic replacement of viruses contained in influenza vaccines is necessary for the annual flu shot to be effective against the evolving nature of influenza viruses.
During the current flu season in the U.S., all influenza vaccines are quadrivalent composition.
The most effective way to prevent the disease is vaccination, says the WHO. Moreover, flu shots provide protection, even when circulating viruses do not exactly match the vaccine viruses.
A recent analysis published on February 22, 2023, and MMWR issued on February 24, 2023, indicate the 2022-2023 flu shot preliminary effectiveness was about 68% protective for pediatric patients and 43% against adult hospitalization.
Breaking flu shot news is posted at PrecisionVaccinations.

