Search API
The U.S. Centers for Disease Control and Prevention (CDC) today published enhanced recommendations for the screening and testing for Hepatitis B Virus (HBV) infection.
As of March 10, 2023, the CDC confirms universal screening of adults for HBV infection is cost-effective compared with risk-based screening and averts liver disease, cirrhosis, and death.
Furthermore, chronic HBV infection is detectable using reliable and inexpensive screening tests.
In addition, although not quantifiable, management of chronic infection through prevention efforts can prevent further transmission to others.
These recommendations consider a more straightforward and less stigmatizing implementation strategy than previous risk-based HBV screening recommendations, says the CDC's Morbidity and Mortality Weekly Report.
The CDC's new recommendations also provide guidance complementary to the 2022 recommendations to vaccinate all adults aged 19–59 years against HBV infection by providing a means to establish immunity, any history of infection, or the need for vaccination to protect from future infection.
Previously, on February 23, 2022, the CDC's Hepatitis Vaccines Work Group presented an HVB vaccine update.
The CDC says effective vaccines to prevent hepatitis B are available in the U.S.

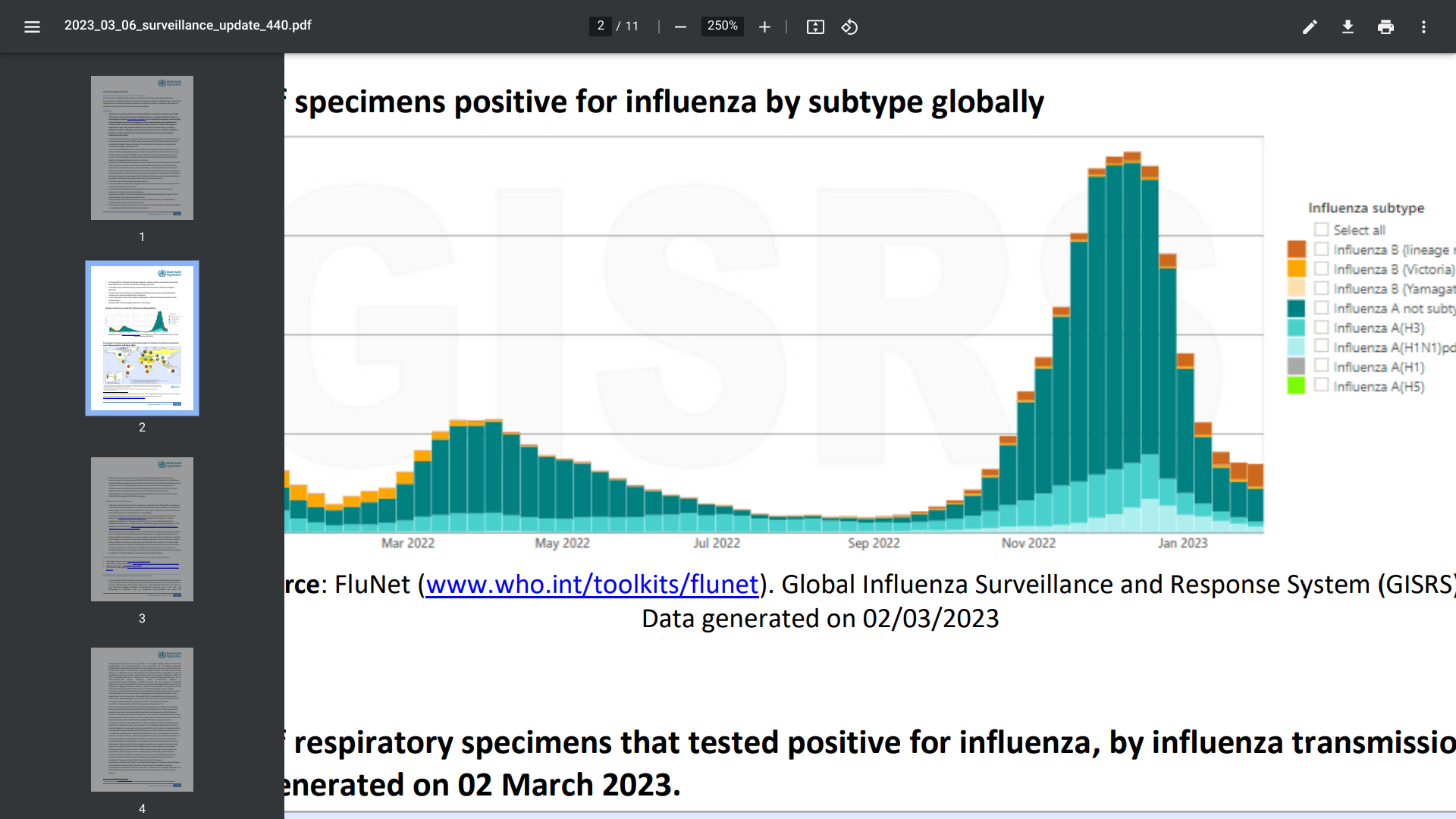
The World Health Organization (WHO) recently published Influenza Update N° 440, influenza activity continued to decrease following a peak in late 2022.
As of March 6, 2023, influenza A viruses predominated, and the proportion of influenza B virus detections increased during the current reporting period.
In the countries of North America, most indicators of influenza activity decreased to levels similar to or below levels typically observed towards the end of the season.
In Europe, overall influenza detections remained stable, and influenza positivity from sentinel sites increased in the most recent week, remaining above the epidemic threshold at the regional level.
Out of 39 countries, 18 reported high or moderate intensity, and over half continued to report widespread influenza case activity.
Other indicators of influenza activity remained stable or decreased in most countries, while a few countries reported increases.
Various types of flu shots remain available in the U.S. and Europe as of March 9, 2022.
As of February 25, 2023, the Centers for Disease Control and Prevention confirmed about 173.26 million flu shots had been distributed in the U.S. during this season.
Additional 2022-2023 flu season news is posted at PrecisionVaccinations.com/Flu.
The World Health Organization (WHO) today announced it would conduct a digital webinar tomorrow discussing Marburg virus vaccine and therapeutic candidates and how research can be integrated during future outbreaks.
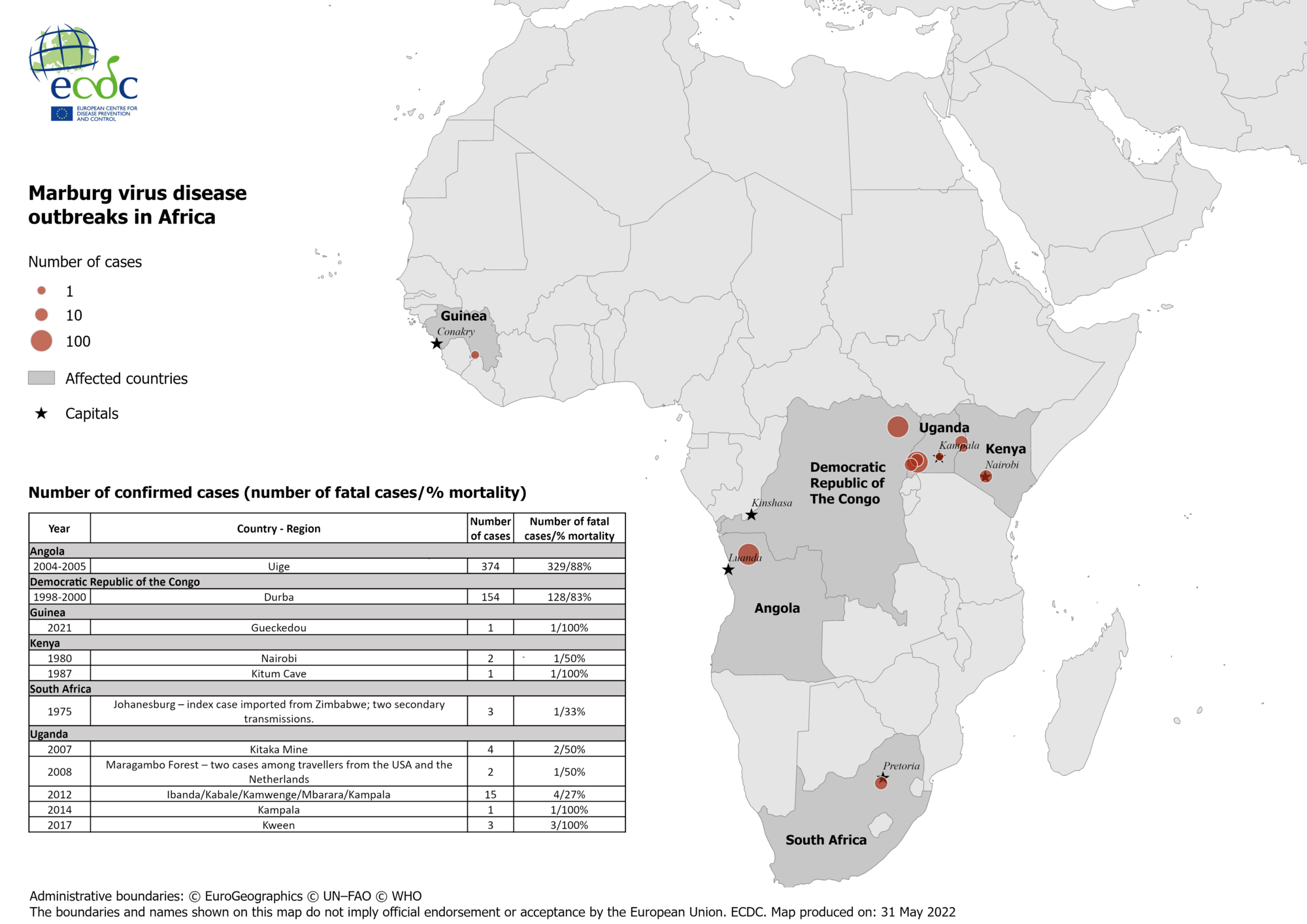
The recent emergence of the Marburg virus (MARV) in Equatorial Guinea (Feb. 2023) and Ghana triggered the assembly of the "MARVAC" consortium in 2022 to facilitate a rapid response to this infectious disease threat.
As of March 9, 2023, several MARV vaccine candidates are conducting early-stage clinical studies.
On March 10, 2023, at 14:00 CET, from this Zoom link, the WHO R&D Blueprint is organizing a consultation with the MARVAC partners and focal points from at-risk countries to discuss this preliminary agenda:
- Outbreak sit rep, including diagnostics and epidemiology,
- Review of core trial protocols for vaccines and therapeutics,
- Panel discussion from at-risk country focal points
Since 1967, Angola, DR Congo, Equatorial Guinea, Cameroon, Germany, Ghana, Guinea, Kenya, Serbia, South Africa, and Uganda have confirmed Marburg cases.
The U.S. Centers for Disease Control and Prevention published the History of Marburg outbreaks.
ModeX Therapeutics, Inc. recently announced it entered into an exclusive worldwide license and collaboration agreement with Merck to develop MDX-2201, a preclinical nanoparticle vaccine candidate targeting Epstein-Barr virus (EBV).
Under the terms of the agreement, ModeX and Merck will jointly advance MDX-2201 to a U.S. FDA Investigational New Drug application filing, after which Merck will be responsible for clinical and regulatory activities and product commercialization.
MDX-2201 is based on ModeX’s ferritin nanoparticle vaccine platform, which can express as many as 24 copies of a recombinant antigen on its surface to enhance the presentation of key virus components and stimulate durable protective immunity.
MDX-2201 presents antigens from four viral proteins involved in viral entry into host cells.
These include a recombinant antigen designed from the proteins gH, gL, and gp42 and an antigen derived from gp350.
By using ModeX’s multi-targeted approach, this combination inhibits infection in two cell types, B cells and epithelial cells, contrasting with efforts previously focused on gp350 alone.
“At Merck, we have a proud legacy of developing vaccines, including several that have the potential to help protect against certain types of cancer,” said Tarit Mukhopadhyay, Ph.D., Vice President, Infectious Diseases and Vaccine Discovery, Merck Research Laboratories, in a press release on March 8, 2023.
EBV is a member of the herpes virus family and is one of the most common human viruses.
EBV can cause infectious mononucleosis and is associated with other illnesses, including specific types of cancer and multiple sclerosis. Unfortunately, there are currently no FDA-approved vaccines or treatments for EBV infection.
However, Moderna Inc. has been conducting a phase 1 clinical study for its mRNA-1189 vaccine candidate.
The viral proteins in mRNA-1189 are expressed in their native membrane-bound form for recognition by the immune system.

Charlotte Elton with EuroNews today reported Ibiza health officials are urging tourists to be on alert for symptoms of a Dengue infection.
The scenic Balearic Island, located off Spain's east coast, has recently experienced several dengue outbreaks.
For example, a message from Germany in 2023 alerted Spain to six cases of native dengue reported between May and November last year.
The Centre for Coordination of Health Alerts and Emergencies (CCAES) recently confirmed one of the potential carriers of dengue is the Aedes albopictus or tiger mosquito.
These mosquitoes are found throughout the Spanish Mediterranean, northern Spain, including France.
From May to December 9, 2022, 272 imported cases of dengue fever were identified in metropolitan France, most from implantation by Aedes albopictus.
In addition to Europe's dengue outbreaks, several countries have reported dengue cases in 2023, including south Florida in the U.S.
Dengue can take up to two weeks to develop, with the illness generally lasting less than a week, says the U.S. CDC.
Health effects from dengue include fever, headache, nausea, vomiting, rash, muscle and joint pain, and minor bleeding. And dengue can become severe within a few hours.
Severe dengue is a medical emergency, usually requiring hospitalization.
Dengue is a vaccine-preventable disease. And there are two dengue vaccines available in specific countries.

When Uber Inc. reintroduced its ride-sharing service in Cancun, Mexico, in 2023, most international travelers were excited to use a known transportation service.
Uber customers have digital access to the same features while traveling at home, including safety features such as 24/7 support, GPS tracking, and emergency assistance.
And after a long day at the beach, Uber delivers Cancun cuisine with just a few taps.
However, according to USA Today reporting on March 6, 2023, local cab services contest Uber's availability.
Earlier this year, the U.S. Department of State issued a security notice regarding incidents involving Uber drivers in Quintana Roo and Cancun.
However, official complaints against Uber drivers do occur, and past disputes between these services and local taxi unions have occasionally turned violent, resulting in injuries to U.S. citizens in some instances, says the State Department.
U.S. citizens are reminded of guidance provided on Travel.State.gov, specifically about using application-based transportation services in Mexico.
From a health perspective, the U.S. CDC recommends several vaccinations before visiting Mexico.
And the dengue virus transmission has been documented in Yucatan, Mexico, since 1979. Dengue is reported in 28 of 32 states in Mexico.
And dengue is an ongoing risk when enjoying Cancun's beaches!
Visitors to areas of risk should protect themselves from dengue infection by preventing mosquito bites.
There are two authorized dengue vaccines, but access requires testing.
Note: This news article is not paid content.

Moderna Inc. president Stephen Hoge commented at a conference on healthcare on March 6, 2023, indicating it would accelerate the approval pathway for its personalized cancer vaccine candidate mRNA-4157/V940.
"At some point, this randomized 150-person Phase IIb study that we ran might — might — be able to become the basis of accelerated approval," Hoge said, according to EndPoint News.
"It's too early to say, but we are hopeful that the data will mature that way."
On December 13, 2022, mRNA-4157/V940, combined with KEYTRUDA, demonstrated a statistically significant and clinically meaningful reduction in the risk of disease recurrence or death compared to KEYTRUDA monotherapy in stage III/IV melanoma patients with a high risk of recurrence following complete resection.
An advantage of Moderna's mRNA platform is that it allows for investigational medicines that combine several different approaches to activate the immune system to attack cancer in a single mRNA therapy.
The U.S. Food and Drug Administration has already issued breakthrough therapy designation for mRNA-4157/V940 ahead of a potential Phase 3 study for the treatment expected in 2023 for adjuvant melanoma, a type of skin cancer.
In 2019, the latest year for which incidence data are available, 88,059 new cases of melanoma of the skin were reported, and 8,092 people died of this cancer in the U.S. Cancer is the second leading cause of death in the U.S., exceeded only by heart disease, says the U.S. CDC.
The CDC's real-time cancer dashboard is available at this link.

The Annals of Internal Medicine today published a systematic review and meta-analysis of three randomized clinical trials in adults with prediabetes.
The U.S. Centers for Disease Control and Prevention (CDC) says prediabetes is a serious health condition where blood sugar levels are higher than normal but not high enough to be diagnosed as type 2 diabetes (T2D).
Published on March 7, 2023, this analysis concluded vitamin D usage effectively decreased the risk of developing TD2.
This data found vitamin D use reduced the risk for diabetes by 15% (hazard ratio, 0.85 [95% CI, 0.75 to 0.96]) in adjusted analyses, with a 3-year absolute risk reduction of 3.3% (CI, 0.6% to 6.0%).
Among participants assigned to the vitamin D group who maintained an intratrial mean serum 25-hydroxyvitamin D level of at least 125 nmol/L (≥50 ng/mL) compared with 50 to 74 nmol/L (20 to 29 ng/mL) during follow-up, cholecalciferol reduced risk for diabetes by 76% (hazard ratio, 0.24 [CI, 0.16 to 0.36]), with a 3-year absolute risk reduction of 18.1% (CI, 11.7% to 24.6%).
And Vitamin D increased the likelihood of regression to normal glucose regulation by 30% (rate ratio, 1.30 [CI, 1.16 to 1.46]).
Furthermore, there was no evidence of a difference in the rate ratios for adverse events (kidney stones: 1.17 [CI, 0.69 to 1.99]; hypercalcemia: 2.34 [CI, 0.83 to 6.66]; hypercalciuria: 1.65 [CI, 0.83 to 3.28]; death: 0.85 [CI, 0.31 to 2.36]).
This study's limitations included 'people with prediabetes do not apply to the general population.'
The CDC says approximately 96 million American adults have prediabetes. However, more than 80% of those with prediabetes don't know they have it.
This study had no external funding, and the researchers did not disclose any industry conflicts of interest.
In May 2022, the U.S. Food and Drug Administration approved Eli Lilly's Mounjaro injection to improve blood sugar control in adults with type 2 diabetes as an addition to diet and exercise. Mounjaro is not indicated for use in patients with type 1 diabetes.
As of March 7, 2023, no FDA-approved vaccine prevents diabetes.