Search API
GSK plc recently announced positive results from a phase 3 clinical trial evaluating its MenABCWY combination vaccine candidate, administered as two doses given six months apart in healthy individuals aged 10-25 years.
GSK's MenABCWY vaccine candidate combines the antigenic components of its licensed meningococcal vaccines, Bexsero (MenB) and Menveo (MenACWY).
All primary endpoints were met, including the non-inferiority of the vaccine candidate for all five Neisseria meningitides serogroups (A, B, C, W, and Y) compared to licensed meningococcal vaccines Bexsero and Menveo in terms of an immune response.
In addition, the vaccine candidate was well tolerated, with a safety profile consistent with Bexsero and Menveo.
Tony Wood, Chief Scientific Officer at GSK, commented in a press release on march 14, 2023, "These statistically significant phase III data are a very encouraging step toward reducing the incidence of meningococcal disease."
"In the U.S., routine use of a 5-in-1 meningococcal vaccine with a two-dose regimen in adolescents at 16 to 18 years of age, just before this disease's incidence peak, could drive significant public health impact."
Invasive meningococcal disease (IMD), a significant cause of meningitis and septicemia, is an uncommon but serious illness that can cause life-threatening complications or even death, typically amongst previously healthy children and adolescents.
Five Neisseria meningitides serogroups (A, B, C, W, and Y) account for nearly all IMD cases worldwide.
Among those contracting meningococcal diseases, one in ten will die, sometimes in as little as 24 hours, despite treatment.
As yet, no licensed combination vaccine offers protection against these serogroups in a single vaccine.
Currently, in the U.S., two separate vaccines needing four injections are required to protect against all five serogroups.
This immunization regimen and low awareness of the disease can lead to sub-optimal immunization coverage rates, particularly for MenB, with an estimated coverage of only about 31% of adolescents in the U.S.
GSK works closely with regulators to review the complete phase III data set, including the supplemental Biologics License Application for Bexsero.
This clinical trial was the confirmatory trial for Bexsero and the phase III trial for MenABCWY.
Detailed results from this phase III trial will be presented in a peer-reviewed publication and at upcoming scientific meetings.
The Government of Canada today confirmed its' main priority continues to be protecting the health and safety of Canadians. Throughout the recent pandemic, decisive actions taken empowered Canada to scale up domestic biomanufacturing capacity, which had been in decline for over 40 years.
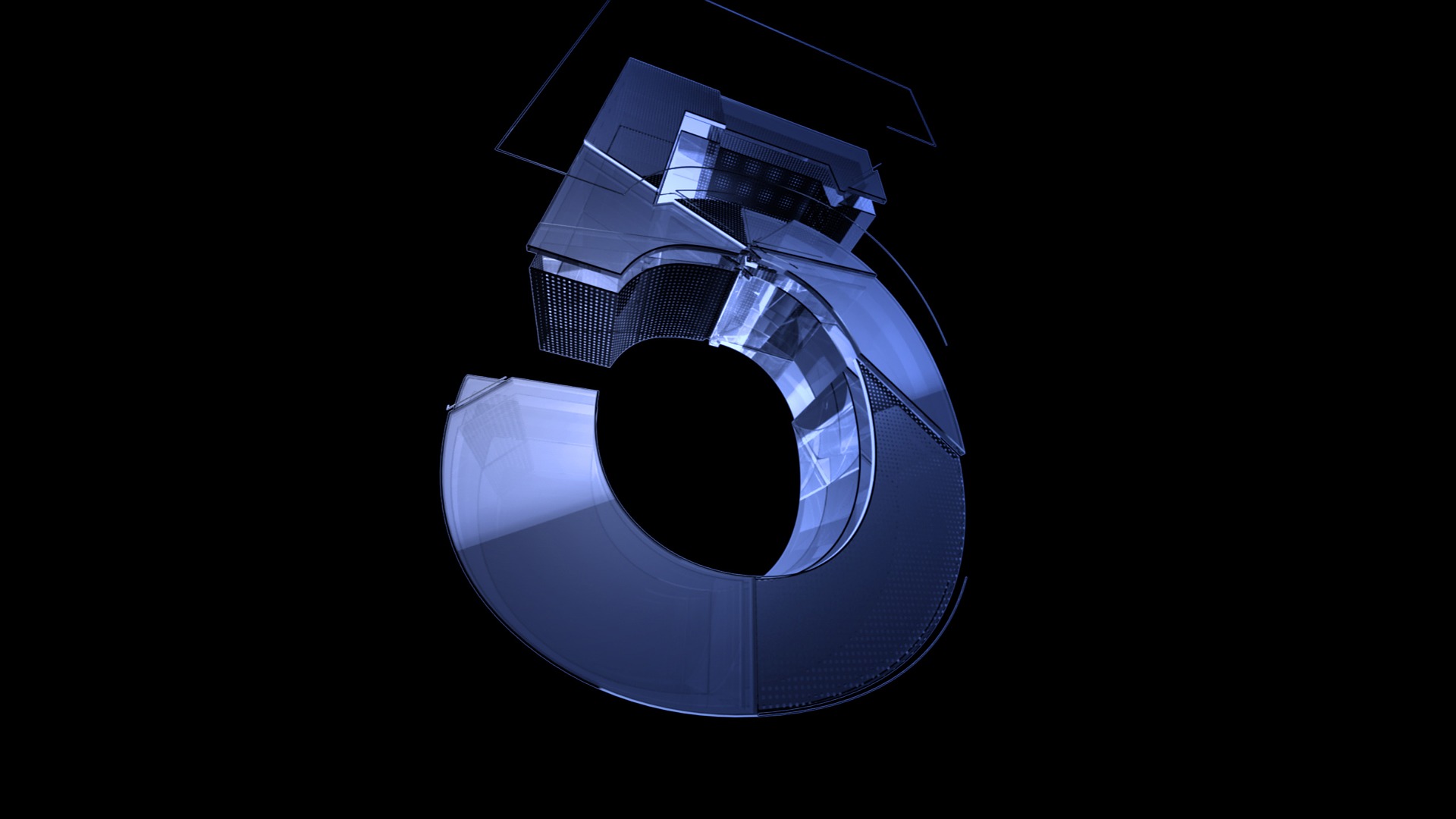
On March 14, 2023, Adam van Koeverden, Parliamentary Secretary to the Minister of Health and to the Minister of Sport, highlighted in a press release an investment of $2 million to create the Canadian Hub for Health Intelligence & Innovation in Infectious Diseases (HI3).
This support is part of a $10 million investment announced on March 2, 2023, for creating five research hubs as part of Stage 1 of the integrated Canada Biomedical Research Fund and Biosciences Research Infrastructure Fund competition.
The University of Toronto (UofT) leads the Canadian Hub for Health Intelligence & Innovation in Infectious Diseases.
The HI3 hub is led by co-directors Jen Gommerman and Scott Gray-Owen, professors of immunology and molecular genetics, respectively, in the Temerty Faculty of Medicine at U of T.
It will focus on advancing the concept of "personalized and precise medicine" to influence the development of vaccines, therapeutics, and other public health interventions.
The U.S. Food and Drug Administration (FDA) presented some good news last week regarding influenza vaccines. The FDA confirmed this season's influenza vaccination provided substantial protection against inpatient, emergency department, and outpatient illnesses among all ages.
On March 7, 2023, Lisa Grohskopf, MD, MPH, with the U.S. Centers for Disease Control and Prevention (CDC), presented to the FDA's Vaccines and Related Biological Products Advisory Committee updated vaccine effectiveness (VE) information through January 2023.
Dr. Grohskopf's presentation highlighted influenza vaccination significantly reduced disease by the following:
- 39% (95%CI: 31, 45) against adult hospitalizations,
- 44% (95%CI: 41, 47) against adult ED or UC visits, and
- VE was observed across age groups and immunocompromised.
Furthermore, this flu season's estimates are higher than VE estimates against hospitalization (25%) and ED or UC visits (25%) from the 2021–22 season.
As of March 15, 2023, the CDC says various flu shots remain available at most clinics and pharmacies in the U.S., and late-season vaccinations are advised for certain at-risk people.
Over 173 million influenza vaccines have already been distributed this flu season.

A recent Lancet Lancet Infectious Diseases analysis concluded that a single dose of Modified Vaccinia Ankara (MVA-BN, Jynneos®) vaccine was very protective against mpox.
Published on March 13, 2023, Dimie Ogoina, with Niger Delta University and colleagues, wrote the 78% vaccine effectiveness reported suggests that a single dose of Jynneos is considered protective against symptomatic mpox only after 13 days post-vaccination.
Furthermore, this analysis and other studies suggest that using a single dose of Bavarian Nordic's Jynneos as pre-exposure prophylaxis is preferable to post-exposure prophylaxis to guarantee protection against symptomatic mpox.
And because people living with HIV have been shown to have a higher risk of breakthrough COVID-19 infections post-vaccination, identifying four of eight breakthrough mpox infections among people living with HIV is noteworthy.
However, the Jynneos vaccine has previously been shown to be immunogenic among adults with a history of AIDS.
Since there are no approved HIV vaccines, co-administration data is unavailable.
Additional Mpox outbreak news is posted at MpoxToday.

Communities in India have voiced strong interest in accessing HIV self-testing, says the World Health Organization (WHO).
The WHO today announced it recommends HIV self-testing (HIVST) as an important approach to address gaps in HIV diagnoses, including among key populations in India.
HIVST can also generate demand for prevention services and facilitate pre-exposure prophylaxis delivery.
The first of the United Nations’ 95-95-95 targets to end the HIV epidemic is for 95% of people living with HIV to know their HIV status by 2025. HIV testing is therefore essential to achieving “the first 95”.
A report launched in New Delhi in 2022 showed HIVST is acceptable to key populations and their partners in India.
In the U.S., clinicians are recommended to screen for HIV infection in all pregnant women, including those who present in labor or at delivery and whose HIV status is unknown.
And screening is endorsed for certain adolescents and adults who are at increased risk of HIV infection.
Globally, 98 countries now have policies supportive of HIVST, and 52 are routinely implemented, yet many countries have not yet introduced HIVST as a routine approach.
Until HIV-preventive vaccine candidates are approved, HIVST is a key component to reducing infections.

Eisai Co., Ltd. today announced that the U.S. Veterans' Health Administration (VHA) is providing coverage of LEQEMBI™ to veterans living with early stages of Alzheimer's disease (AD).
As of March 13, 2023, VHA healthcare professionals meeting the criteria set forth by the VHA can prescribe LEQEMBI to veterans who fit the VHA's standards and the U.S. Food and Drug Administration's (FDA) current label.
The FDA-approved LEQEMBI under the accelerated approval pathway in January 2023, and was launched in the U.S. on January 18, 2023.
LEQEMBI is a humanized immunoglobulin gamma 1 monoclonal antibody directed against aggregated soluble (protofibrils*) and insoluble forms of amyloid beta (Aβ),
Treatment with LEQEMBI should only be initiated in patients with mild cognitive impairment or mild dementia stage of disease and confirmed presence of Aβ pathology, the population in which treatment was initiated in clinical trials.
After the first infusion, 38% of LEQEMBI-treated patients had transiently decreased lymphocyte counts to <0.9 x109/L compared to 2% on placebo, and 22% of LEQEMBI-treated patients had transiently increased neutrophil counts to >7.9 x109/L compared to 1% on placebo.
Furthermore, there are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied.
Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial.
LEQEMBI is not a vaccine but is therapeutically administered via infusion.
In the event of an infusion-related reaction, the infusion rate may be reduced, or the infusion may be discontinued, and appropriate therapy initiated as clinically indicated. Prophylactic treatment with antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs, or corticosteroids prior to future infusions may be considered.
The FDA has not approved an Alzheimer's disease vaccine as of March 2023.
Parents living in St. Thomas, Elgin, and Oxford counties in Ottawa were recently advised to be alert to respiratory symptoms, which are particularly dangerous in young children.
Symptoms of the vaccine-preventable disease pertussis start with a runny nose or nasal congestion, sneezing, mild cough, and mild fever.
Southwestern Public Health, which is located between Detroit and Toronto, announced on March 8, 2023, parents and guardians should keep themselves and their children up to date with the pertussis vaccine after a recent dramatic rise in cases in the region.
"Our region has seen 82 confirmed cases of pertussis between January 2022 and February 28, 2023. This represents about 40% of the provincial total from that time period."
"Combine this with the number of children who are unvaccinated or under-vaccinated, and I am concerned in particular for the youngest members of our community," says Dr. Ninh Tran, Medical Officer of Health for Southwestern Public Health, in a related press release.
Pertussis, commonly known as whooping cough, is a vaccine-preventable disease.
This vaccine is routinely administered to children along with protection from polio, tetanus, and diphtheria (DTaP).
In the U.S., DTaP vaccines such as Boostrix are offered at clinics and pharmacies.
Pertussis is very contagious and spreads via droplets from the noses and mouths of those who are infected.
The cough, which can last anywhere from 2 – 8 weeks, gets progressively worse and may lead to vomiting or trouble breathing and coughing up mucous. It can often be recognized by the loud "whooping" sound that occurs when the child is inhaling after a coughing spell.
Untreated pertussis in infants can lead to hospitalization, brain damage, and death.
Furthermore, new research indicates an expecting mother can take action to protect her future child.
According to an Original Investigation published by JAMA Pediatrics in February 2023, maternal Tdap vaccination reduces pertussis burden in infants (2 months).
"I have two asks of our local parents."
"The first is that you make yourself familiar with the symptoms of pertussis and seek medical care if your child has these symptoms."
"It can be treated with antibiotics, and after five days on the treatment, the person can no longer spread the disease to others."
"Second, please contact your family health care provider or Southwestern Public Health to get your child's routine vaccinations up to date."
"The vaccine is free, and we have openings in our clinics throughout the month of March," adds Tran.
Ottawa residents requiring a public health vaccination clinic appointment can book online at www.swpublichealth.ca/booking.