Search API
Vaxxinity, Inc. today announced that the first subjects had been dosed in a randomized, double-blind, placebo-controlled Phase 1 clinical trial of VXX-401, an investigational vaccine designed to lower low-density lipoprotein (LDL) cholesterol, a known factor contributing to heart disease.
Heart disease remains the leading cause of death globally, claiming over 18 million deaths yearly.
VXX-401 is designed to induce robust, long-acting antibodies against PCSK9 to lower LDL cholesterol.
The multicenter Phase, 1 dose-escalation trial, aims to enroll 48 subjects aged 18 to 75 years with LDL cholesterol between 2.59 and 4.89 mmol/L.
The trial evaluates safety, tolerability, and immunogenicity (as measured by serum anti-PCSK9 antibody titers).
LDL cholesterol levels will measure the pharmacodynamics of the immune response, an established model of PCSK9 inhibition in hypercholesterolemia. This study was last updated on March 16, 2023.
VXX-401 was designed using Vaxxinity's proprietary synthetic peptide vaccine platform and is being developed to treat hypercholesterolemia.
The platform is designed to harness the immune system to convert the body into its own natural "drug factory," stimulating the production of antibodies.
"This is an exciting milestone for VXX-401 and Vaxxinity in our pursuit to vaccinate the world against heart disease with a preventative option that is convenient and accessible, addressing an unmet need to combat the leading global cause of death," said Mei Mei Hu, Chief Executive Officer of Vaxxinity, in a press release on March 20, 2023.
"PCSK9 antibody therapies are well-tolerated and effective, but huge, unmet patient need remains."
"In order to solve the problem of heart disease, the world needs a scalable, accessible technology that can reach the hundreds of millions, if not billions, of people at risk."
"With an LDL-lowering vaccine, we can offer an option that's cost-effective, safe, convenient, long-acting, and deployable."

Despite recent improvements in diagnostic tools, chikungunya outbreaks in Africa are probably underreported, stated a U.S. CDC Early Release Dispatch, Volume 29, Number 4—April 2023.
During 2019–2020, a large-scale chikungunya outbreak occurred in Djibouti City, the capital city of the Republic of Djibouti, located in the Horn of Africa.
Djibouti is a semi-arid country bordered by Eritrea, Somalia, and Ethiopia. In this region, the primary vector of the chikungunya virus (CHIKV) is the Aedes aegypti mosquito.
The chikungunya outbreak remained limited (attack rate 2.1%) but was followed by a dengue outbreak.
These researchers found clinical features helpful but insufficient to discriminate between chikungunya and dengue viruses.
However, CHIKV blood samples on blotting paper have been described as a field method for detecting arboviruses, routinely used in the French Armed Forces when deployed in Africa.
In this study, the researchers used blood samples on blotting paper to detect the emergence of CHIKV and monitor the course of the outbreaks.
Blotting paper provided a robust method for blood sampling and transport to a reference laboratory, making it possible to confirm 90% of the arboviral diagnoses.
We recommend blotting paper as a field tool to detect and monitor arboviral epidemics remotely, wrote these researchers.
Various countries, like Paraguay, are reporting chikungunya outbreaks in remote areas.
As of March 21, 2023, no chikungunya vaccines are authorized in Africa, Europe, or the U.S.
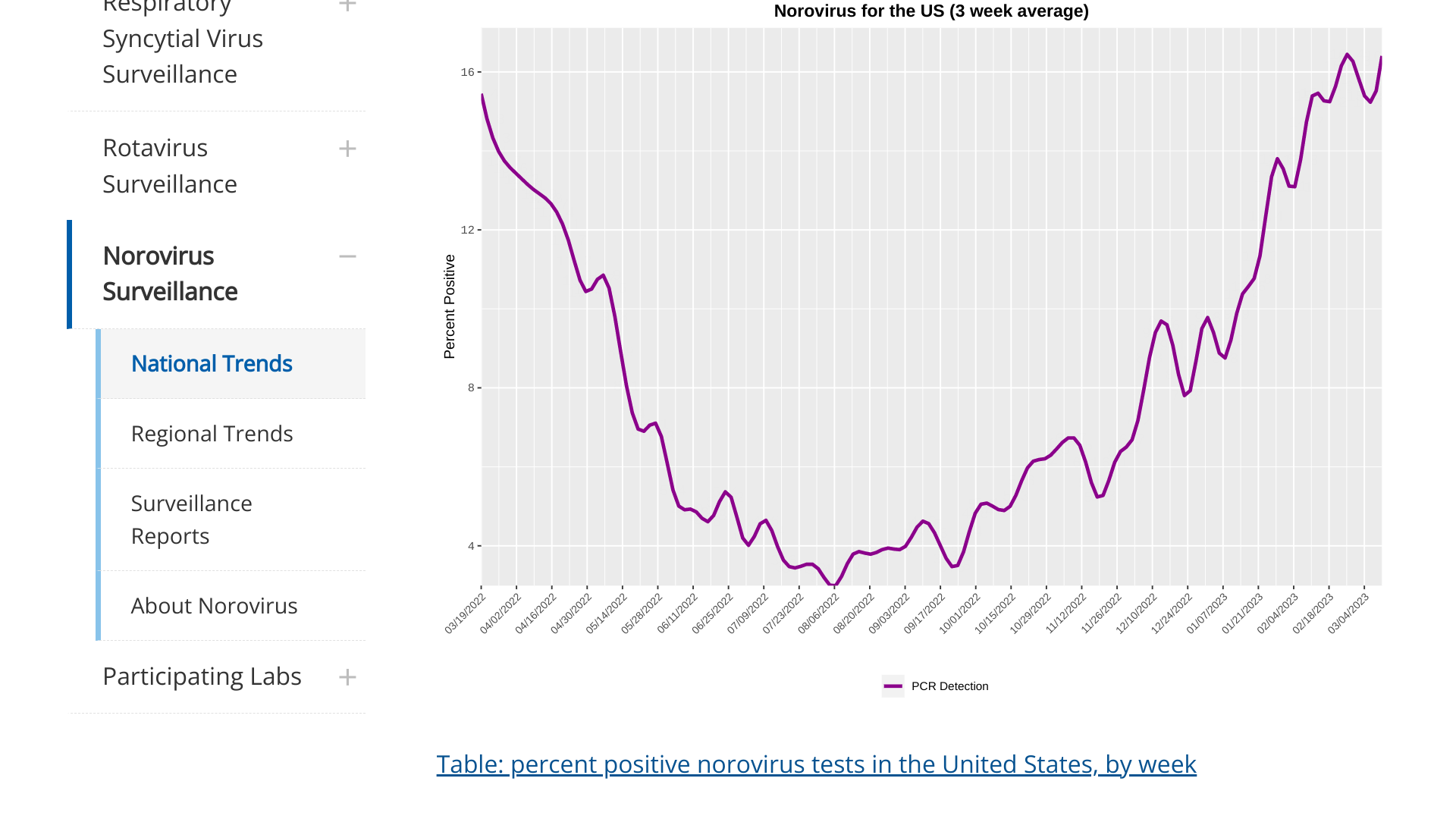
The National Center for Immunization and Respiratory Diseases recently reported data indicating cases of norovirus are spiking in the United States.
Norovirus is a very contagious virus that causes vomiting and diarrhea.
As of March 15, 2023, the 3-week moving average is reaching a new peak since the norovirus outbreak began in August 2022.
Each of the four regions has displayed similar trends, with the Northeast registering the steepest uptick.
In the U.S., cases of norovirus occur most frequently during late fall, winter, and early spring.
According to the U.S. Centers for Disease Control and Prevention (CDC), people can get norovirus illness many times in their life because there are many different types of noroviruses.
Infection with one type of norovirus may not protect you against other types.
The CDC says it is possible to develop immunity to specific types. But, it is not known exactly how long immunity lasts.
This may explain why so many people of all ages get infected during norovirus outbreaks. About 80% of children will experience a norovirus infection within one year of birth.
As of March 21, 2023, there are no approved norovirus vaccines in the U.S., Europe, or the U.K.
The peer review journal The Lancet Infectious Diseases published the results from a recent study examining prospective respiratory syncytial virus (RSV) surveillance data to assess the geotemporal prevalence of RSV A and B and functionally characterize the effect of the nirsevimab binding-site substitutions identified between 2015 and 2021.
Nirsevimab (Beyfortus®), an extended half-life monoclonal antibody (mAbs) to the RSV fusion protein, has been developed to protect infants for an entire RSV season.
This AstraZeneca and Sanofi-funded observational analysis concluded on March 17, 2023, nirsevimab binding site was highly conserved, and escape variants were rare and have not increased over time.
The U.S. Food and Drug Administration (FDA) initially approved an injectable mAbs therapy for children in 1998.
Beyfortus has been granted various regulatory approvals.
As of March 21, 2023, neither the FDA nor the European Medicines Agency approved an RSV vaccine candidate for children or older adults.
However, various authorizations are expected in 2023.

Regeneron Pharmaceuticals, Inc. and Sanofi today announced that the European Commission (EC) had approved Dupixent® in the European Union to treat severe atopic dermatitis in children aged six months to 5 years old who are candidates for systemic therapy.
With this approval on March 21, 2023, Dupixent is the first and only targeted medicine indicated to treat these children in Europe and the U.S.
Dupixent is a fully human monoclonal antibody injection administered under the skin at different injection sites.
Atopic dermatitis is a chronic type 2 inflammatory skin disease. Between 85% and 90% of patients first develop symptoms before five years of age, which can often continue through adulthood.
“Watching an infant or young child grapple with the debilitating and wide-reaching impacts of severe atopic dermatitis is heartbreaking,” said Korey Capozza, MPH, Founder and Executive Director of Global Parents for Eczema Research, in a press release.
“I’ve personally witnessed how this chronic skin disease can disrupt the lives of entire families when left uncontrolled. However, intervening with effective treatments during infancy and early childhood can help manage the challenging impact this disease has on children and their families during such formative years.”
Severe atopic dermatitis may also significantly impact the quality of life of young children and their caregivers. Treatment options in this age group are primarily topical corticosteroids, which can be associated with safety risks and may impair growth when used long-term.
The approval is based on data from a Phase 3 trial evaluating Dupixent every four weeks (200 mg or 300 mg based on body weight) plus low-potency primarily topical corticosteroids (TCS) or TCS alone (placebo) in 162 children aged six months to 5 years with moderate-to-severe atopic dermatitis.
At 16 weeks, Dupixent improved skin clearance and reduced overall disease severity and itch compared to placebo in the overall enrolled population. However, in a subset of those with severe atopic dermatitis, patients randomized to Dupixent (n=63) experienced the following compared to placebo (n=62) at 16 weeks:
- In addition, 46% of patients achieved 75% or greater improvement in overall disease severity compared to 7% treated with placebo, a co-primary endpoint.
- 14% of patients achieved clear or almost clear skin compared to 2% treated with placebo, a co-primary endpoint.
- 55% average reduction in overall disease severity from baseline compared to 10% with placebo.
- 42% average reduction in itch from baseline compared to a 1% increase with placebo.
Dupixent also improved sleep quality, skin pain, and health-related quality of life compared to placebo in both the overall and severe populations. In addition, long-term efficacy data showed the clinical benefit at 16 weeks was sustained through 52 weeks.
The most common side effects across indications include injection site reactions, conjunctivitis, conjunctivitis allergic, arthralgia, oral herpes, and eosinophilia.
Dupixent is currently approved for one or more indications in more than 60 countries, including Europe, the U.S., and Japan. More than 600,000 patients are being treated with Dupixent globally.

Sweden's Public Health Agency recently reported several severe influenza cases have occurred in Orebro County, located west of Stockholm. Complications can occur in connection with influenza infection, but this outbreak is unusual.
The spread of influenza A and B is estimated to continue throughout the country as of March 17, 2023.
During week #10, there was roughly the same number of cases of influenza A (385 cases) as influenza B (364 cases).
So far, eleven newly admitted patients with laboratory-confirmed influenza have been reported in intensive care during week 10, of which six with influenza A and five with influenza B.
The current investigation aims to assess whether there are more influenza B cases with serious complications than expected and whether there is any common contributing cause. Therefore, the Public Health Authority has asked other infection control units to investigate whether there are similar cases in other regions.
Of the 21 countries that reported sentinel primary care specimen influenza virus positivity above the 10% epidemic threshold, France, Hungary, Romania, and Slovenia reported activity above 40%.
So far, sequencing of clinical specimens from severe cases in Sweden has identified B/Victoria viruses belonging to subgroup V1A.3a.2, which is the dominant influenza type B virus circulating across Europe and the northern hemisphere 2022-23 and 2023-24 influenza vaccine strain (B/Austria/1359417/2021-like virus).
When traveling abroad, the U.S. Centers for Disease Control and Prevention suggests getting a second flu shot if visiting an area with influenza activity.
Various flu shots are available in the U.S. at health clinics and travel pharmacies.

The Socialist Republic of Vietnam's Health Ministry today announced it has requested for visitors arriving from African countries with Marburg virus disease outbreaks to be monitored for three weeks while in-country.
The ministry also requested samples be taken in suspected cases while at local airports.
"This is a highly dangerous disease," the ministry noted, according to local media on March 20, 2023.
The request was made as the highly contagious disease killed nine people in Equatorial Guinea and could spread further into other African regions and Spain.
As of March 20, 2023, no approved Marburg vaccines or antivirals exist.
Vaccitech plc today announced topline interim data from the HPV001 Phase 1b/2 clinical trial of VTP-200 heterologous prime-boost immunotherapy in women with low-grade cervical human papillomavirus (HPV) lesions.
It is estimated that approximately 291 million women worldwide are carriers of HPV DNA.
Data from the first 58 women enrolled who reached their 6-month timepoint in the HPV001 placebo-controlled study were reviewed internally, and the trial will continue as planned to the 12-month primary endpoint. Immunogenicity results showed high responses, defined as an average greater than 1,000 spot-forming units per million peripheral blood mononuclear cells in an ELISPOT assay, especially to the E1, E2, and E6 antigens.
VTP-200 was generally well-tolerated with no product-related grade 3 unsolicited events and no product-related SAEs.
"These interim data are a promising step in the right direction, and we look forward to seeing the final data in early 2024," said Bill Enright, CEO of Vaccitech, in a press release on March 20, 2023.
"Currently, people with persistent HPV infections have no treatment options until they develop high-grade lesions. Being told to return for a repeat cervical screening every 6 to 12 months without a treatment option can be frustrating and anxiety-provoking."
"VTP-200 is intended to treat HPV infections, potentially before the virus causes these high-grade lesions."
VTP-200 is being developed as a potential non-invasive treatment for persistent high-risk HPV infections and associated pre-cancerous lesions.
Persistent genital HPV infection is responsible for almost all cases of cervical pre-cancerous lesions, which can lead to cervical carcinoma.
Over 95% of cervical cancers are caused by HPV infection.
The American Cancer Society predicts that in 2022, approximately 14,100 new cases of invasive cervical cancer were diagnosed in the U.S., with over 4,280 women dying from the disease.
In the U.S., various HPV vaccines are authorized for women and men and available at most clinics and clinical pharmacies in March 2023.


