Search API
The National Veterinary Services Laboratories (NVSL) today announced the presence of Highly Pathogenic Avian Influenza (HPAI) in a striped skunk recovered from Carson County, located east of Amarillo in the Texas panhandle.
As of March 21, 2023, this is the first confirmed case of HPAI in mammals in Texas.
HPAI is a highly contagious virus that transmits efficiently among wild and domestic birds. The virus can spread directly between animals and indirectly through environmental contamination.
Since the H5 clade 2.3.4.4b. appeared in North America in January 2022, over 6,000 H5N1 detections in wild birds, by 47 states (14 states within 30 days), leading to the loss of over 58 million birds as of March 17, 2023.
For mammals, current data shows transmission occurs primarily through the consumption of infected animal carcasses, though mammal-to-mammal transmission does not appear sustainable.
Other mammal species confirmed with HPAI in the U.S., Canada, Central Ameria, and South America include foxes, raccoons, bobcats, opossums, mountain lions, and black bears.
Because of the ease of transmission, the TPWD recommends that wildlife rehabilitators also remain cautious when intaking wild animals with clinical signs consistent with HPAI and consider quarantining animals to limit the potential for HPAI exposures to other animals within the facility.
Currently, the transmission risk of avian influenza from infected birds to people remains low.
But the public should take basic protective measures, such as wearing gloves, face masks, and handwashing if contact with wild animals cannot be avoided.
Furthermore, the U.S. government has approved avian influenza vaccines should human-to-human transmission occur since the annual flu shot is not effective against this type of influenza.

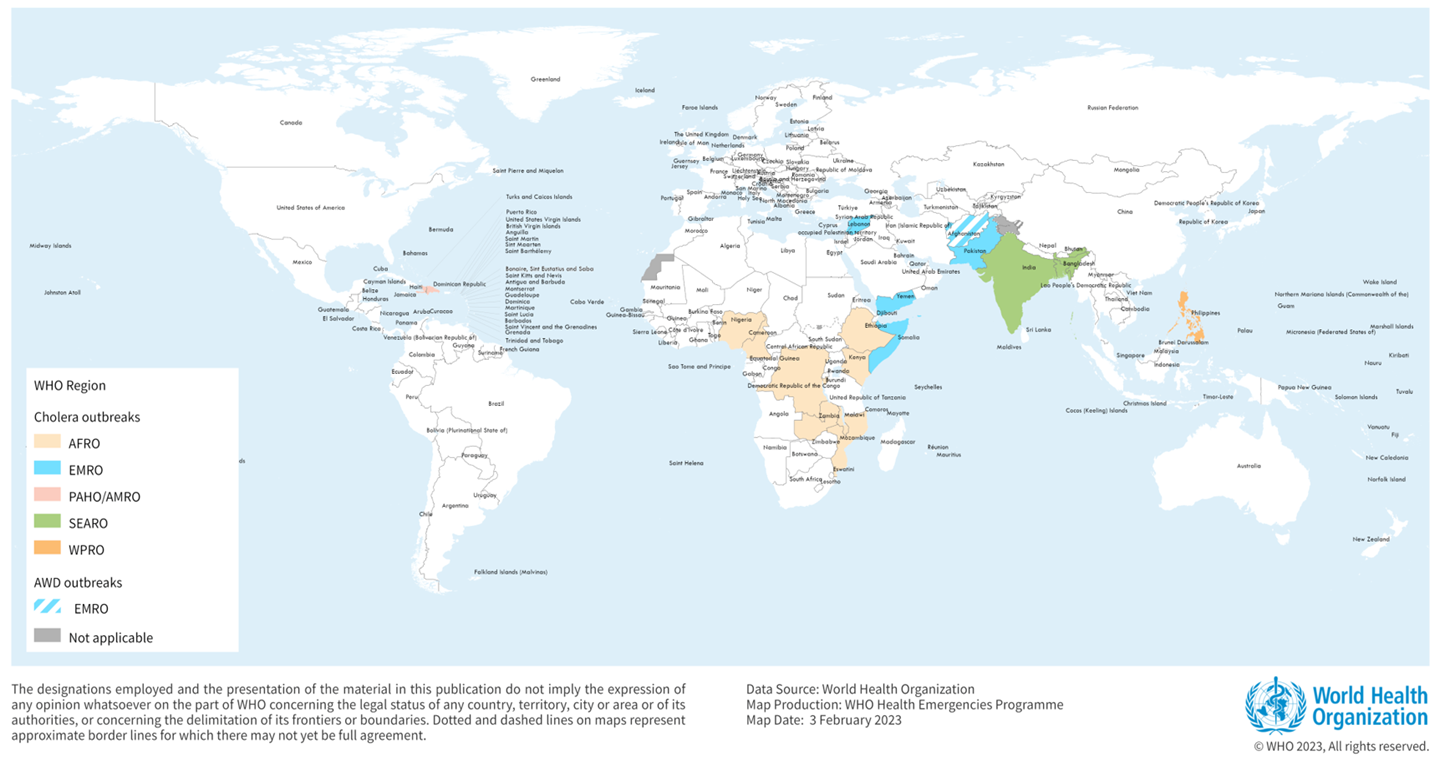
The world is facing an upsurge in Cholera, even touching countries that have not had the disease in decades, announced the World Health Organization (WHO) on World Water Day.
Years of progress against this age-old disease have disappeared, stated the WHO on March 22, 2023. In the past months, the world has seen a resurgence of Cholera.
Last year, as many as 30 countries experienced outbreaks, and we continue to see a worrying geographic spread into 2023.
While the situation is unprecedented, the lesson to draw is not new: safe drinking water, sanitation, and hygiene are the only long-term and sustainable solutions to ending this cholera emergency and preventing future ones.
The global cholera situation is concerning, but the historic United Nations Water Conference began in New York. The Global Task Force for Cholera Control appeals to countries and the international community to channel that concern toward concrete action.
Nearly all cholera cases reported in the U.S. are acquired during international travel, says the U.S. Centers for Disease Control and Prevention (CDC).
For example, the CDC confirmed eight travelers infected with Cholera arrived in the U.S. from Pakistan, Iraq, and Bangladesh in 2022.
In 2023, various countries have confirmed cholera outbreaks.
As of March 22, 2023, cholera vaccines, such as Valneva SE's DUKORAL® oral, inactivated Cholera, and ETEC Diarrhea vaccine, have been approved and are available in certain countries.
A recent article published by eBioMedicine discussed how the Zika virus leads to olfactory disorders in mice by targeting olfactory ensheathing cells.
On February 3, 2023, this article confirmed Zika virus (ZIKV) is an emerging arbovirus of the genus flavivirus associated with congenital Zika syndrome (CZS) in newborns.
Clinical symptoms, including intellectual disability, speech delay, coordination or movement problems, and hearing and vision loss, have been well-documented in children with CZS.
However, whether ZIKV can invade the olfactory system (ability to detect odors) and lead to post-viral olfactory dysfunction (PVOD) remains unknown.
These researchers demonstrated that neonatal mice infected with ZIKV suffer transient olfactory dysfunction when they reach puberty.
Moreover, ZIKV mainly targets olfactory ensheathing cells (OECs) and exhibits broad cellular tropism colocalizing with small populations of mature/immature olfactory sensory neurons (mOSNs/iOSNs), sustentacular cells and horizontal basal cells in the olfactory mucosa (OM) of immunodeficient AG6 mice.
ZIKV infection induces strong antiviral immune responses in the olfactory mucosa and olfactory bulb tissues, resulting in the upregulation of proinflammatory cytokines/chemokines and genes related to the antiviral response.
'Our results demonstrate that the olfactory system represents a significant target for ZIKV infection and that PVOD may be neglected in CZS patients,' concluded these researchers.
The authors declared that they have no conflict of interest.

The U.S. Centers for Disease Control and Prevention (CDC) published Dispatch, Volume 26, Number 12, on November 19, 2020; Susceptibility of Raccoon Dogs for Experimental SARS-CoV-2 Infection, which is inserted below:
Raccoon dogs might have been intermediate hosts for severe acute respiratory syndrome coronavirus (SARS-CoV-1) in 2002–2004.
Our experimental study demonstrates that raccoon dogs are susceptible to SARS-CoV-2 infection. However, in our research, raccoon dogs had only subtle clinical signs.
Additionally, we found evidence of viral replication and tissue lesions in only the nasal conchae, which can transmit the virus to direct in-contact animals.
Increasing evidence supports the potential of carnivore species, including farmed fur animals, to become infected by SARS-CoV-2.
This transmission could eventually cause zoonotic infections in humans.
Our results indicate that affected farms might be reservoirs for SARS-CoV-2.
Thus, efficient and continuous surveillance should target susceptible animals, including raccoon dogs, especially in China, which is a key player in global fur production.
We also need to initiate large-scale epidemiologic field studies with historical samples that might elucidate the role of farmed animals in the current pandemic.
This article was preprinted. Dr. Freuling is a research scientist at the Friedrich-Loeffler-Institut.
Previously, the World Organisation for Animal Health reported on March 31, 2020, in Hong Kong that neither of the two dogs which were positive for SARS-CoV-2 showed clinical signs of COVID-19 infection.

Alzamend Neuro, Inc. today announced the completion of the clinical portion of its Phase IIA multiple ascending dose ("MAD") study for dementia related to Alzheimer's.
The topline data is expected to be disclosed in June 2023.
AL001 is a novel lithium-delivery system; it is a lithium-salicylate-L-proline engineered ionic cocrystal under development as an oral treatment for patients with dementia-related to mild, moderate, and severe cognitive impairment associated with Alzheimer's.
AL001 can potentially deliver the benefits of marketed lithium carbonate while mitigating or avoiding current toxicities associated with lithium.
"We strongly believe that AL001's patented ionic cocrystal technology could potentially provide clinicians with a major improvement over current lithium-based treatments and may constitute a means of treating over 40 million American suffering from Alzheimer's, bipolar disorder, MDD, and PTSD," said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on March 22, 2023.
"We look forward to reporting topline data in June 2023 and further advancing clinical development of this promising potential therapeutic."
Having completed the clinical portion of the MAD study, the resulting pharmacokinetic and statistical data are undergoing evaluation of the safety and tolerability of AL001 under multiple-dose, steady‑state conditions.
This characterizes the maximum tolerated dose in healthy young and elderly subjects and subjects diagnosed with mild to moderate Alzheimer's.
Potentially safe and effective doses will be determined for deployment in planned subsequent Phase IIA clinical trials involving Alzheimer's, bipolar disorder, MDD, and PTSD subjects.
Lithium has been well-characterized for safety and is approved/marketed in multiple formulations for bipolar affective disorders.
AL001 lithium ascending dosing for the MAD cohorts tested incremental fractions of the usual lithium exposure for the treatment of bipolar affective disorder, with the target lithium dose for Alzheimer's treatment expected at a level that will not require therapeutic drug monitoring.
In each of the multiple healthy young/elderly and Alzheimer's cohorts, consisting of 6 active and 2 placebo patients each (as per randomization), multiple ascending doses were administered three times daily for 14 days under fasted conditions up to tolerability/safety limits that included the highest dose permitted per protocol.
As of March 22, 2023, there are no approved vaccines targeting Alzheimer's disease.
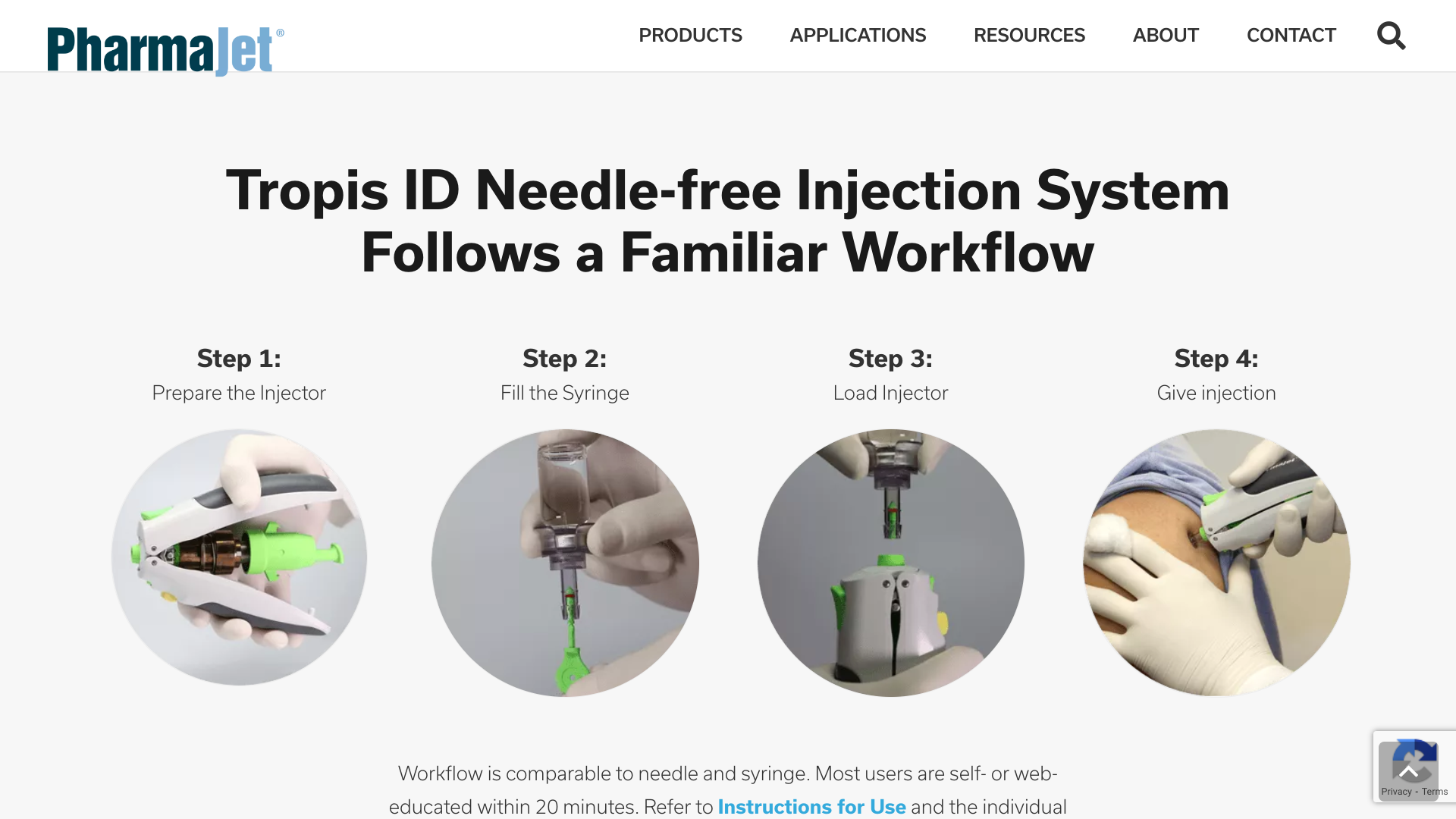
PharmaJet® today announced that its partner, Scancell, reported positive results from their Phase 1 COVIDITY clinical trial. The trial was conducted at the University of Cape Town Lung Institute in South Africa to assess the safety and immunogenicity of their COVID-19 DNA candidate vaccines, SCOV1 and SCOV2.
The results from the trial were highly encouraging, inducing neutralizing antibody and T cell responses with no safety concerns. Administration with PharmaJet's devices was well received by study participants.
This new set of human data adds to the growing evidence indicating that this modern needle-free administration technology is an increasingly viable option to enhance plasmid DNA vaccine immune response.
The vaccines were exclusively administered using the PharmaJet Tropis® and Stratis® needle-free precision delivery systems.
Professor Lindy Durrant, Chief Executive Officer, Scancell, commented in a press release on March 21, 2023, "We are encouraged by these results."
"The trial validates that AvidiMab®-modified immunotherapies boost immune responses and PharmaJet's Needle-free Injection Systems are effective in delivering our ImmunoBody®-generated drug candidate."
"Our plans are to include PharmaJet Needle-free precision delivery systems in future trials with our immuno-oncology projects."
PharmaJet Needle-free precision delivery Systems provide increased vaccine effectiveness, a preferred patient and caregiver experience, and a proven path to commercialization.
The Stratis® System has U.S. FDA 510(k) marketing clearance, CE Mark, and WHO PQS certification to deliver medications and vaccines either intramuscularly or subcutaneously.
The Tropis® System has CE Mark and WHO PQS certification for intradermal injections.
They are both commercially available for global immunization programs.
Note: This news article is not paid content.