Search API
The journal ScienceDirect recently published results from an early-stage, innovative Zika virus vaccine candidate, OraPro-Zika.
On April 6, 2023, this study addressed several challenging features attributed to vaccinology.
First, developing a thermostable vaccine negates the need for a "cold chain." And second, the potential to deliver the vaccine orally.
Having demonstrated that iosBio's OraPro-Zika vaccine provided a measurable efficacy in the murine models, they performed a challenge study in NHP to investigate the potential clinical utility.
In this setting, enteric-coated capsules were given orally, negating the need for stomach acid neutralization.
Upon vaccination, a significant rise in anti-Zika IgG was evident after the first dose concordant with our murine model.
Furthermore, the level of IgG was similar to that in convalescent serum from NHP previously infected with the Zika virus.
Most strikingly, in challenge studies, convalescent and OraPro-Zika vaccinated animals showed no evidence of replicative Zika virus. In contrast, the placebo-vaccinated cohort showed full-blown infection, which modeled the disease pathology as it was rectified by day 8 of the study.
While we hypothesize the immune protection in our model systems is potential via a mucosal root, our study is limited and does not define cellular mechanisms or sIgA.
Nonetheless, IgG and challenge studies remain the gold standard for vaccine efficacy.
In summary, these researchers showed that an orally administered Ad5- vaccine encoding genes for the Zika envelope (Env) and NS1 proteins induced a specific immune response that reduced Zika infection in both murine and NHP models and remained thermally stable for over 109 days at 25 °C.
This preliminary study with OraPro-Zika suggests oral administration of a non-replicating adenovirus vector protects against challenge and warrants further investigation to establish a mode of action.
These authors declared various industry relationships, and Innovate UK supported the study.
Updated May 10, 2023 - Reassigned domain.

The U.S. Centers for Disease Control and Prevention (CDC) today reissued its Watch - Level 1, Practice Usual Precautions regarding dengue outbreaks in Asia and the Pacific Islands.
The CDC stated that on April 17, 2023, the listed countries reported higher-than-usual dengue cases, and travelers visiting these countries may be at increased risk.
Because Dengue is a severe disease spread by mosquito bites, all travelers to risk areas should prevent mosquito bites by using an EPA-registered insect repellent, wearing long-sleeved shirts and long pants outdoors, and sleeping in an air-conditioned room or room with window screens or under an insecticide-treated bed net.
The CDC says Dengue can take up to 2 weeks to develop, generally lasting less than a week.
Additionally, visitors to these countries should speak with a healthcare provider regarding dengue vaccines, such as Dengvaxia and Takeda's QDENGA®.
Vax-Before-Travel posts other dengue outbreak news.
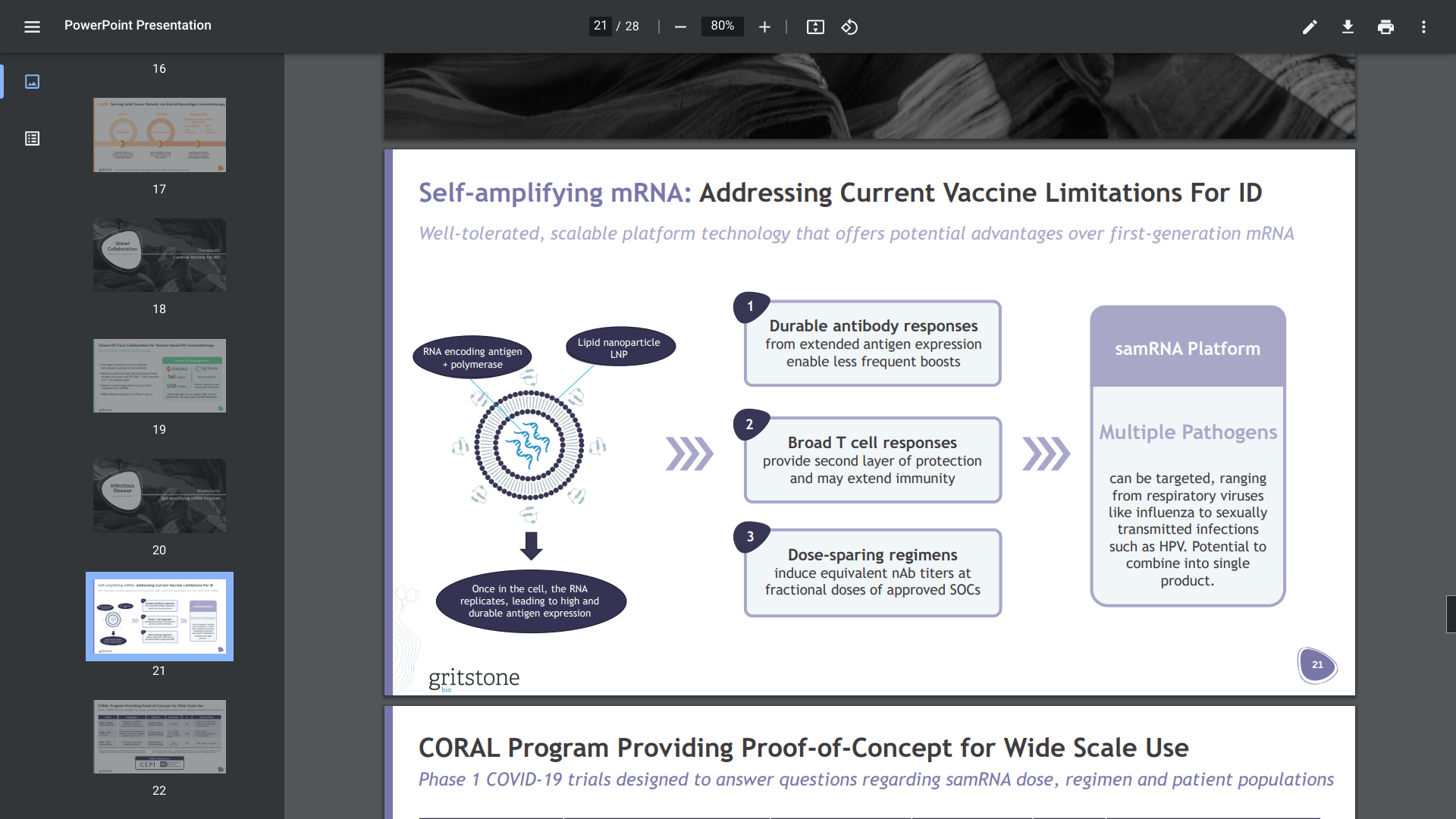
Gritstone bio, Inc. today presented 6-month follow-up data from its ongoing Phase 1 CORAL-CEPI and CORAL-BOOST studies, which are evaluating the company's self-amplifying mRNA (samRNA) vaccine candidates against SARS-CoV-2, at the 33rd European Congress of Clinical Microbiology & Infectious Diseases (ECCMID).
"The results shared today demonstrate that regardless of whether and/or how the immune system was previously exposed to SARS-CoV-2, our samRNA vaccine candidates are driving robust antibody titers that are sustained for at least six months in healthy adults," said Karin Jooss, EVP and Head of R&D at Gritstone bio, in a press release on April 17, 2023.
"Current vaccines against COVID-19 have demonstrated susceptibility to loss of immunity over time, posing a greater burden on individuals and our health systems."
"Developing a next-generation vaccine that drives more durable and broad neutralizing antibodies against variants of concern not included in the vaccine – both of which are demonstrated in these results - could serve as a key factor in delivering long-term, variant-proof immune protection."
"The results shared today further support our hypothesis that samRNA, unique from mRNA due to several distinct characteristics, could serve as a widely applicable next-generation vaccine platform technology against SARS-CoV-2 and beyond."
Gritstone's CORAL program applies an infectious disease approach, which aims to drive B cell and T cell immunity using samRNA against SARS-CoV-2.
The program serves as proof of concept for applying Gritstone's platform against coronaviruses and other infectious diseases. The Bill & Melinda Gates Foundation, the U.S. NIAID, and the Coalition for Epidemic Preparedness Innovations support it.
SAB Biotherapeutics announced today that the U.S. Food and Drug Administration (FDA) had granted Breakthrough Therapy Designation to SAB-176, an investigational therapeutic, for post-exposure prophylaxis for Type A and Type B influenza illness in high-risk patients, including those who have antiviral resistant strains.
SAB-176 is being developed for several influenza indications.
The FDA's Breakthrough Therapy designation confirms that the multi-epitope targeting modality of SAB-176 has a clear differentiation vs. monoclonal antibodies (mAbs) that bind to a single epitope.
And SAB's treatment can sustain its efficacy over viral mutations and prevent or reduce the risk of emerging treatment-resistant influenza strains.
Virus evolution driven by vaccines or treatments is a serious challenge, and the use of therapeutics can create "escape mutants" or versions of a virus that have changed to escape pressure on virus survival driven by antiviral treatment, whether it is a small molecule or mAbs modality, wrote the company.
SAB recently announced that the FDA had granted Fast Track designation to SAB-176.
The company had also received FDA guidance and regulatory alignment on advancing SAB-176 into the next development phase by initiating a Phase 2b clinical trial.
"Influenza continues to pose considerable health concerns in the U.S. and globally. This Breakthrough Therapy designation signifies an important step forward in our fight against this disease," said Eddie Sullivan, Ph.D., co-founder, President & CEO of SAB Biotherapeutics, in a press release on April 18, 2023.
"We are proud that based on generated preclinical and clinical evidence, SAB-176 has received both Breakthrough and Fast Track designations, a combination rarely seen."
The FDA's Breakthrough Therapy designation process is designed to expedite the development and reviewing a medicine intended to treat a serious or life-threatening condition. Preliminary clinical evidence indicates that the drug may substantially improve over current therapies on a clinically significant endpoint(s).
Products that qualify for Breakthrough Therapy designation receive more benefits than Fast Track products.
Precision Vaccinations post influenza vaccines news for April 2023.

The Lancet recently published results from an analysis that assessed COVID-19 pandemic policies and behaviors in the U.S.
These researchers revealed where a person lived indicated COVID-19 risk.
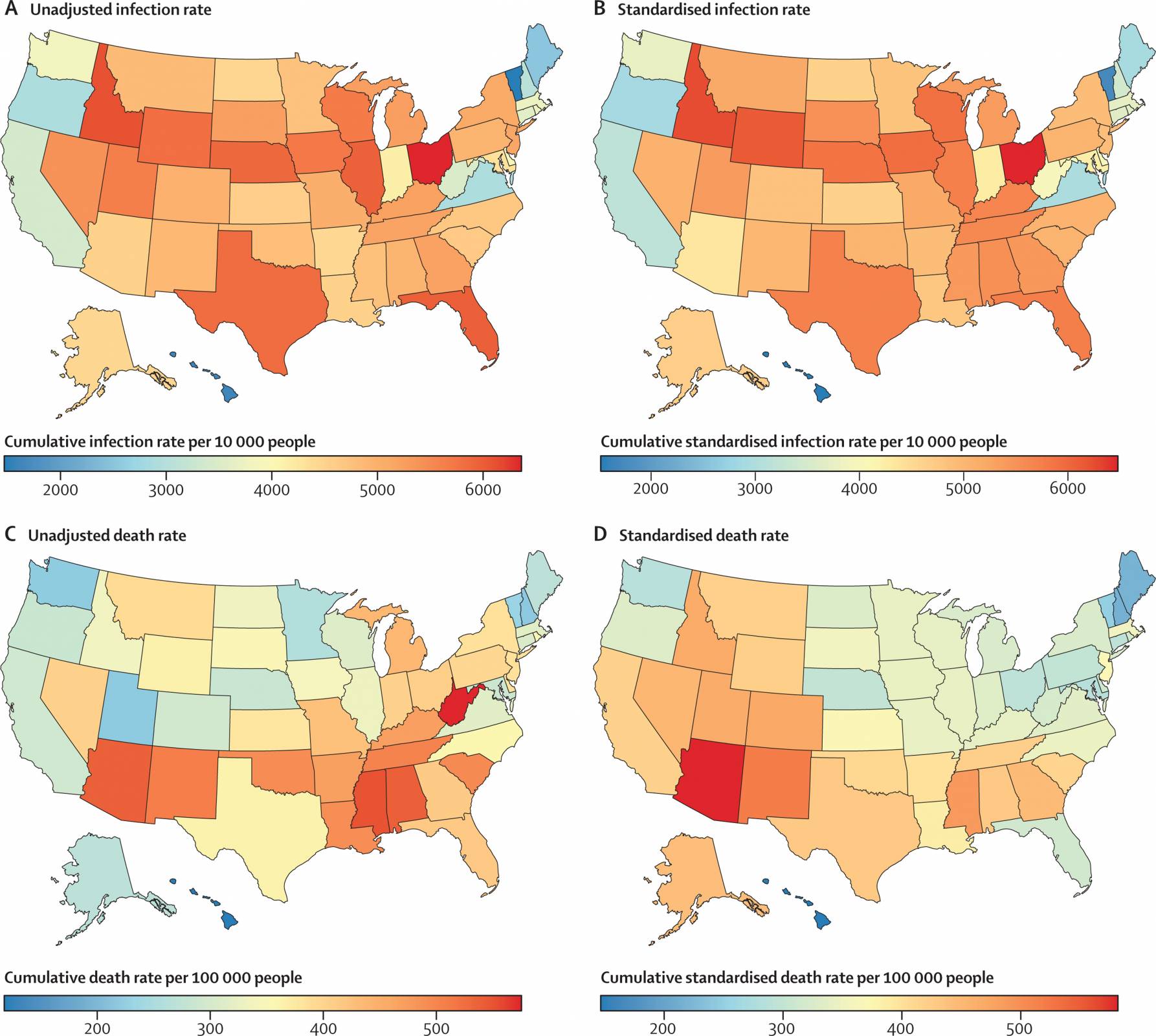
Published on March 23, 2023, this analysis found nearly four-fold differences that existed across states in COVID-19 death rates, even when standardized for factors such as age and comorbidities, suggesting that lower death rates were achievable.
The states with the lowest standardized COVID-19 death rates were Hawaii, New Hampshire, Maine, Vermont, and Maryland, which are not confined to a single geographical region.
And the states and territories with the highest standardized cumulative death rates were Arizona (581 per 100 000 [509–672]), Washington, DC, New Mexico, Mississippi, and Colorado.
In summary, these researchers stated that the policy mandates and protective behaviors adopted during this pandemic effectively reduced SARS-CoV-2 infections.
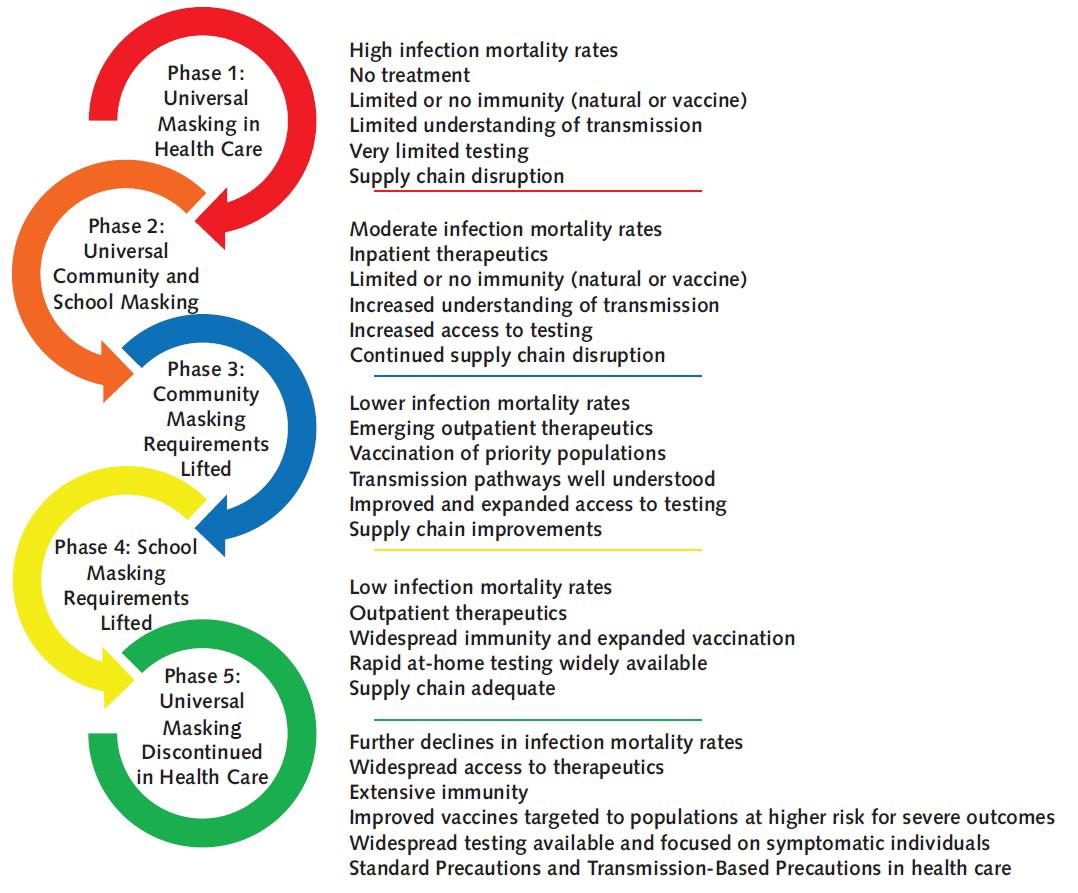
The Annals of Internal Medicine today published an opinion article that confirmed during the COVID-19 pandemic, the expanded use of facemasks as part of “universal masking” for healthcare personnel (HCP), patients, and visitors in healthcare settings was implemented to reduce the risk for morbidity and mortality associated with the spread of a novel virulent pathogen.
However, the context and conditions of the pandemic have changed dramatically, and evidence-based public health policy should also adapt in response.
The time has come to manage SARS-CoV-2 as we generally manage other endemic respiratory viruses in healthcare settings through correct and consistent application of Standard Precautions and Transmission-Based Precautions.
Moving away from universal masking policies should be accompanied by reconsidering other pandemic-era strategies, such as asymptomatic testing and resource-intensive contact tracing.
In conclusion, this article published on April 18, 2023, stated .... Interactions between humans and pathogens are inherently dynamic.
Therefore, they are constantly evolving, and we have achieved significant advancements in preventing and managing SARS-CoV-2 since the pathogen was initially identified in 2019.
In recognition of these achievements, the time has come to deimplement policies inappropriate for an endemic pathogen when the expected benefits of such policies are low.
Universal masking in health care is a policy whose time has come and gone ... for now.
Face mask research is posted by Coronavirus Today.
Prof Mojisola Christianah Adeyeye, Director-General of the Federal Republic of Nogeria's National Agency for Food and Drug Administration And Control (NAFDAC), today announced it granted registration approval for the R21/Matrix-M™ vaccine.
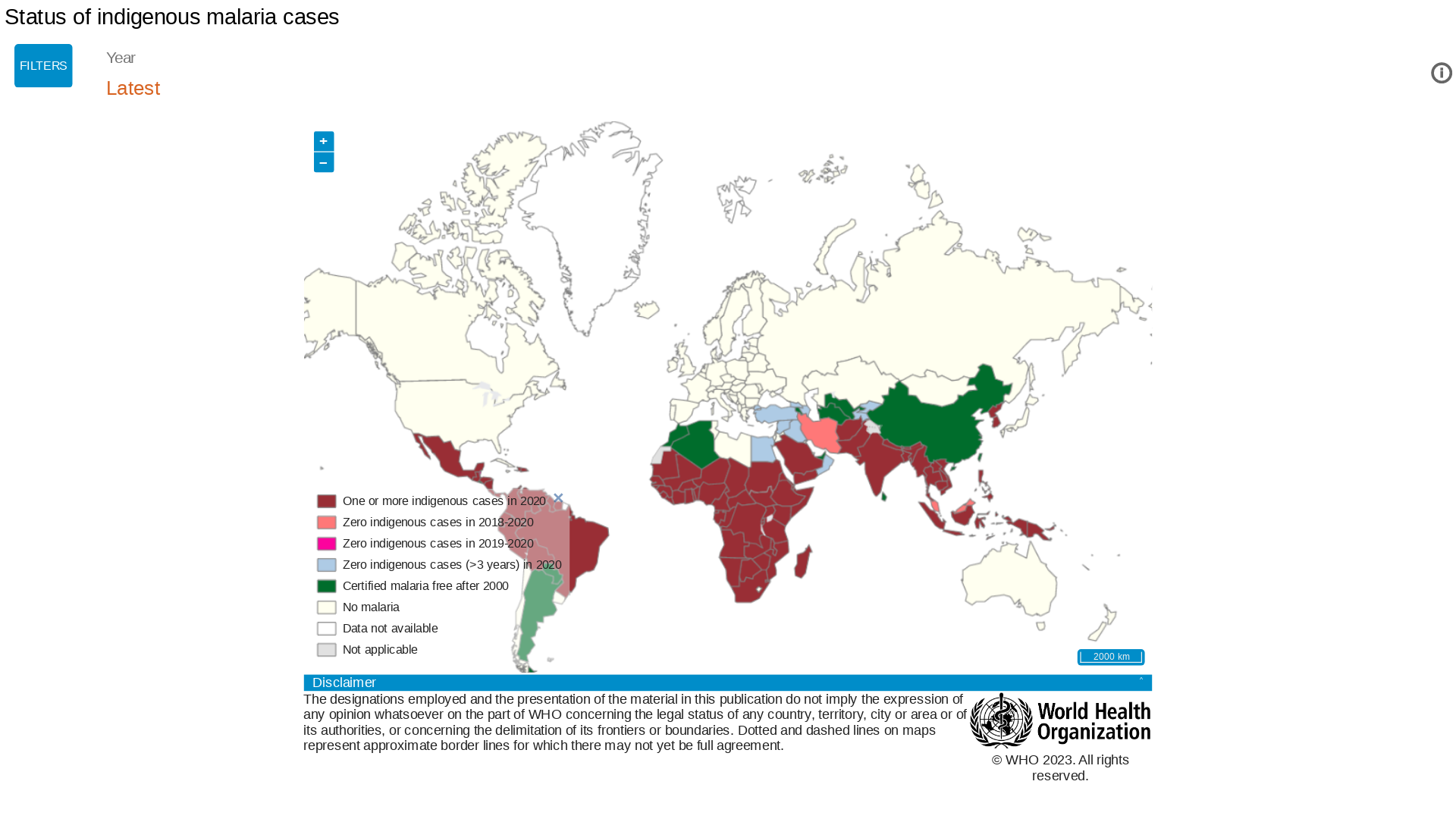
The NAFDAC approval on April 17, 2023, is essential since the WHO African Region continues to carry a disproportionately high share of the global malaria burden.
For example, in 2021, the Region was home to about 95% of all malaria cases and 96% of deaths.
And malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
Furthermore, the prevalence of malaria parasitemia in Nigerian children under five is about 23%.
The R21 was created by the University of Oxford Jenner Institute in England, is manufactured by Serum Institute of India Pvt. Ltd., and includes Novavax AB proprietary saponin-based Matrix-M adjuvant.
In addition to malaria, the U.S. CDC has issued various Travel Advisories regarding disease outbreaks in Nigeria.
Vaccine-preventable diseases such as yellow fever, measles, and polio are health risks when visiting Nigeria in 2023.
The Nigerian National Agency for Food and Drug Administration and Control (NAFDAC) today announced its approval for the R21/Matrix-M™ Malaria Vaccine manufactured by India's Serum Institute of India Pvt. Ltd.
The Marketing Authorization Holder is Fidson Healthcare Ltd.
During a press briefing on April 17, 2023, Prof Mojisola Christianah Adeyeye, Director-General NAFDAC, said malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
According to the 2021 World Malaria Report, Nigeria had the highest number of global malaria cases (27%) and the highest number of related fatalities (32%) in 2020
This is the second authorization for R21/Matrix-M this month, following the Republic of Ghana.
GSK's Mosquirix™ RTS,S recombinant malaria vaccine was authorized in 2020.
Additional malaria vaccine and outbreak news are posted at Vax-Before-Travel.