Search API
GSK plc today announced that the European Medicines Agency's Committee for Medicinal Products for Human Use had adopted a positive opinion by consensus recommending approval of GSK's respiratory syncytial virus (RSV) vaccine candidate for the prevention of lower respiratory tract disease caused by RSV in adults aged 60 years and older.
If approved, AREXVY™ RSV OA candidate has the potential to be the first RSV vaccine available to help protect older adults.
The European Commission's final decision is expected by July 2023.
This is the first time an RSV vaccine candidate for adults has gained a positive opinion, one of the final steps in the marketing authorization procedure before approval by the European Commission.
As of April 27, 2023, no RSV vaccines or specific treatments are currently available for older adults in Europe or the U.S.
RSV is a common contagious virus affecting the lungs and breathing passages. RSV causes over 270,000 hospitalizations and approximately 20,000 in-hospital deaths in adults aged 60 years and older each year in Europe.
However, according to recent information, RSV's intensity may have returned to normal in the U.S.
The U.S. CDC's Morbidity and Mortality Weekly Report, published on April 7, 2023, presented the seasonality of RSV in the U.S. from 2017–2023.
The CDC reported the 2022–23 RSV season started later than the 2021–22 season but earlier than the prepandemic seasons, suggesting a return toward prepandemic seasonality.
For updated information, the CDC's RSV-NET interactive dashboard displays trends and comparisons of RSV-associated hospitalizations in various demographic groups and seasons.

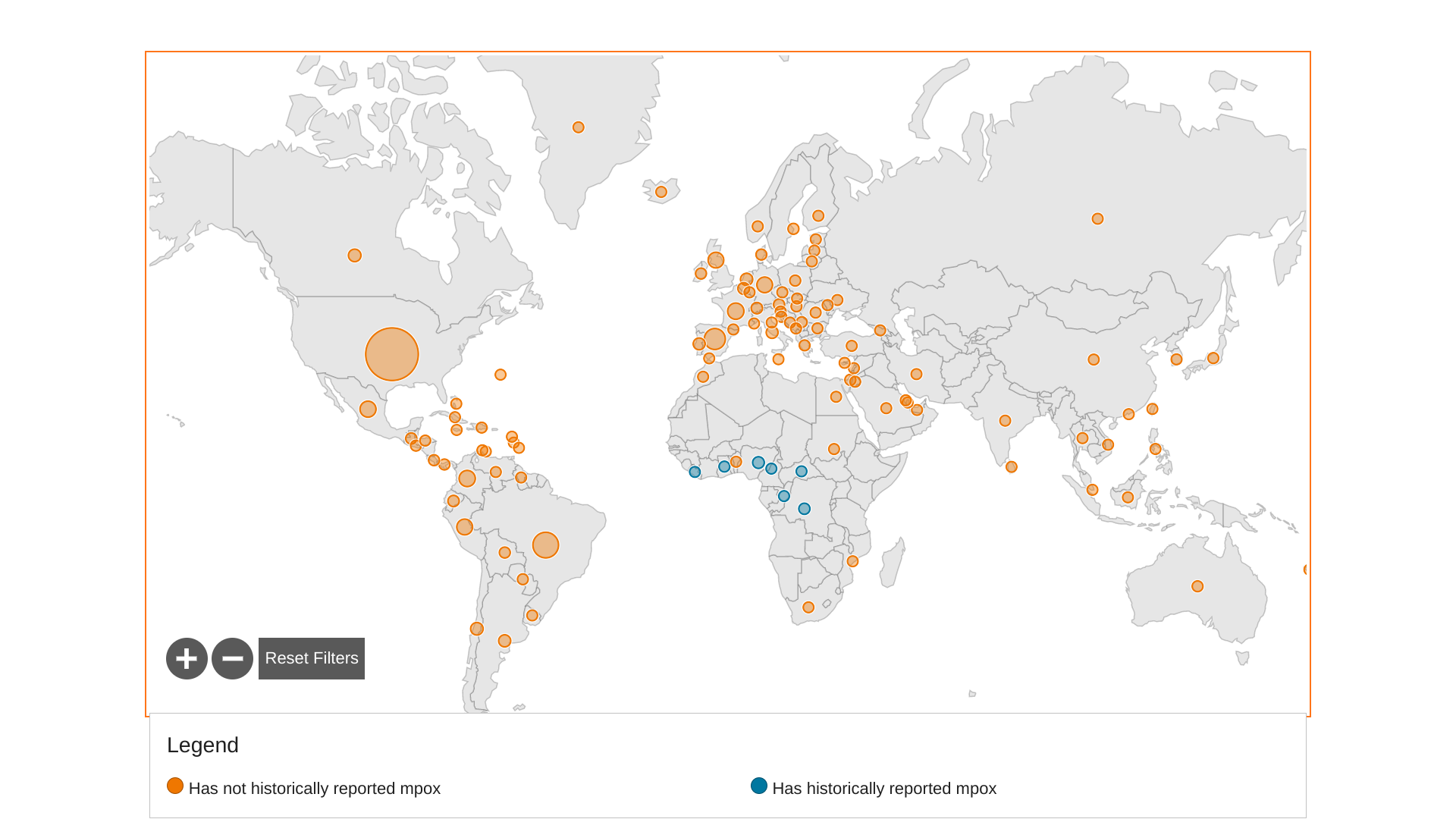
The World Health Organization Africa Region recently reported Mpox cases in the Central Africa Republic, the DRC, Ghana, Liberia, and Nigeria.
As of April 16, 2023, fourteen newly confirmed Mpox cases were reported, resulting in a 5.6% increase.
And three new Mpox-related fatalities were reported.
The top three African countries with the most confirmed Mpox cases include Nigeria, 833, the DRC 450, and Ghana, 124. Together, the three countries have reported 93.7% of all confirmed Mpox cases in Africa.
To alert international travelers of potential health risks when visiting Africa, the U.S. CDC has published Watch - Level 1, Practice Usual Precautions notices for the DRC and Nigeria.
Globally, over 86,000 Mpox cases have been reported by 110 countries since the outbreak began in May 2022.
While the Mpox outbreak has receded in the U.S., the CDC recommends Bavarian Nordic's JYNNEOS® (MVA-BN) vaccination when visiting at-risk countries in 2023.
Additional Mpox outbreak research is posted at Precision Vaccinations.
Based on a measles outbreak declaration by American Samoa Government, travelers from the Territory (including infants aged six months) must be vaccinated with a measles-containing vaccine before entering Samoa.
Being fully vaccinated indicates completing the required doses, which should have been completed 14 days before traveling.
These islands are located in the South Pacific Ocean, northeast of Fiji.
And Aiono Prof Alec Ekeroma, Samoa's Director General of Health, stated in a Notice that effective May 1, 2023, a legitimate vaccine certificate/note is required before boarding. In addition, a hard copy must be presented at check-in and upon arrival into Samoa.
Vaccine certificates in electronic form stored on phones or other electronic devices must have a QR code. In addition, they must be a Health Authority Approved Certificate/Card of the country where the vaccination occurred.
Furthermore, upon arrival in Samoa, all passengers must wear face masks at all times.
And visitors must submit to a nasal pharyngeal swab for PCR testing upon request by Health Officials at Samoa's airport.
As of April 26, 2023, the U.S. CDC recommends various routine and travel vaccinations before visiting America Samoa, an unincorporated territory of the U.S.
Prior to the recent pandemic, about 20,000 people visited America Samoa annually.
Bloomberg Law News recently reported that Chinese foreign ministry spokeswoman Mao Ning confirmed beginning April 29, 2023, China will no longer require visitors to provide a negative PCR test result.
Instead, visitors can show negative rapid antigen test results, and airlines will not be required to check for proof.
China previously eliminated testing requirements for countries such as New Zealand and Malaysia.
In 2023, the U.S. Centers for Disease Control and Prevention (CDC) began requiring a negative COVID-19 test result or proof of recovery from the virus for all travelers aged two years and older to the U.S. on flights originating from the People's Republic of China (PRC),
As of April 26, 2023, the U.S. CDC recommends various routine and travel vaccinations before visiting the PRC.
Previously, the U.S. Department of State issued a notice stating 'reconsider travel' to the PRC, including the Special Administrative Regions of Hong Kong and Macau, due to arbitrary enforcement of local laws.
The U.S. Centers for Disease Control and Prevention (CDC) confirmed today an outbreak of Lassa fever in several states in the Federal Republic of Nigeria.
On April 24, 2023, the CDC reissued a Watch - Level 1, Practice Usual Precautions alert in reaction to the Nigeria Centre for Disease Control and Prevention reporting over 150 Lassa fever-related fatalities in April 2023.
The CDC says visitors to Nigeria should avoid contact with rodents, food, and material that could be contaminated with rodent urine or droppings.
Furthermore, travelers to Nigeria should seek medical care immediately if they develop (during or after travel) fever, chills, headache, fatigue, bleeding, trouble breathing, vomiting, facial swelling, or pain in the chest, back, and abdomen.
Urgency is essential since treatment with antiviral medicine is most effective during the early stages of illness.
Unfortunately, there are no Lassa fever vaccines as of April 25, 2023.
However, travel vaccines are available for Nigeria's other disease outbreaks in 2023, such as polio, yellow fever, and measles.
HilleVax, Inc. today announced the completion of enrollment of the NEST-IN1 clinical trial for children, with over 3,000 subjects enrolled in six countries.
NEST-IN1 is the company’s ongoing Phase 2b trial for HIL-214, its investigational virus-like particle-based vaccine candidate for preventing moderate-to-severe norovirus-related acute gastroenteritis in infants (AGE).
This study was last updated on April 7, 2023. Topline safety and clinical efficacy data from NEST-IN1 are expected in the first quarter of 2024.
“I am excited to announce the completion of enrollment of our NEST-IN1 study, which brings us one step closer to topline results in the first quarter of 2024,” said Rob Hershberg, MD, Ph.D., Chairman and CEO of HilleVax, in a press release on April 25, 2023.
“There are no approved vaccines for norovirus, which results in approximately 700 million cases of acute gastroenteritis and 200,000 deaths per year.”
Norovirus is a very contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus, says the U.S. CDC.
Globally, norovirus is estimated to result in approximately 700 million cases of AGE and 200,000 deaths per year, resulting in over $4 billion in direct health system costs and $60 billion in societal costs per year.

The BMJ today published a study based on U.S. VA data that concluded among people at high risk of progression to severe COVID-19-19, molnupiravir (Lagevrio™) use within five days of SARS-CoV-2 virus infection may be a viable approach to reduce the risk of post-acute sequelae of SARS-CoV-2 (PASC).
Led by VA Saint Louis Health Care System researchers, this study found molnupiravir was also associated with reduced risk of PASC in people who had not received a covid-19 vaccine, had received one or two vaccine doses, and had received a booster dose, and in people with primary SARS-CoV-2 infection and reinfection.
The researchers wrote they don't know whether this study's findings would apply to people without risk factors for severe COVID-19.
As of April 25, 2023, Lagevrio is approved or authorized for use in more than 25 countries, including the United States.