Search API
In the United States, there remains a considerable burden of disease attributed to serotypes not included in currently approved pneumococcal conjugate vaccines.
To address this need, Pfizer Inc. today announced that the U.S. Food and Drug Administration (FDA) had approved PREVNAR 20® for the prevention of invasive pneumococcal disease (IPD) caused by the 20 Streptococcus pneumoniae serotypes contained in the vaccine in infants and children six weeks through 17 years of age.
And for preventing otitis media in infants six weeks through five years of age caused by the original seven serotypes contained in PREVNAR®.
"Today's FDA approval of our vaccine, PREVNAR 20, now offers parents the ability to help protect their children against 20 pneumococcal serotypes in circulation, which represent the majority of pneumococcal disease in U.S. infants and children," said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a press release on April 27, 2023.
"This important PREVNAR 20 approval builds on more than 20 years of real-world impact with PREVNAR and PREVNAR 13, safety data, and effectiveness, highlighting Pfizer's leadership in developing groundbreaking pneumococcal conjugate vaccines to help protect infants and their families from life-threatening infections."
Pneumococcal vaccine news is posted by Precision Vaccinations.

A National Academies committee recently assessed the value of community-level wastewater surveillance in controlling infectious diseases beyond Covid-19, wrote a Perspective published by the NEJM on April 20, 2023.
Nearly 80% of U.S. households are connected to municipal wastewater collection systems that contain biological waste, including discharged pathogens, such as poliovirus, in New York.
To better understand this innovative, ubiquitous method of detecting the prevalence of infectious diseases, listen to an interview with Profs. Michelle Mello and Guy Palmer focused on wastewater surveillance.
The 14:19 audio interview is accessible at this link.

In the shadow of COVID-19, the stage has been set for a global resurgence of one of the most contagious pathogens, measles.
Viruses are again flourishing, with measles poised for global resurgence, says William Moss, MD, director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health.
For example, India has recently reported 61,562 measles cases.
According to the U.S. Centers for Disease Control and Prevention (CDC), measles is a highly contagious, vaccine-preventable disease. About 90% of unprotected people will become infected when exposed to the measles virus.
Measles cases worldwide increased by about 80% during 2022 compared with 2021.
Recent measles outbreaks in Ohio and Kentucky have brought this concern back to the U.S.
In this Q&A written by Joshua Sharfstein, adapted from April 26, 2023, the Public Health On Call episode, Moss speaks with Josh Sharfstein, MD, about how significant setbacks in global vaccine coverage over the past few years have seeded the deadly threat of a measles resurgence.
While wearing a face mask offers some protection from the highly transmittable virus, the CDC prioritizes vaccination.
Measles is a vaccine-preventable disease, with vaccination services offered at most clinics and community pharmacies in the U.S.
The Florida Health Department reported as of April 22, 2023, there had been 63 travel-associated dengue cases and two locally acquired dengue cases confirmed in 2023.
Most of these dengue cases have been confirmed in greater Miami, Florida.
In 2022, 903 travel-associated and 68 locally-acquired dengue cases were reported in Florida.
Overall, 2.8 million dengue cases were reported by 46 countries in the Americas in 2022, representing a two-fold increase from 2021.
From a protection perspective, two dengue vaccines have been authorized in various countries since October 30, 2022,
The World Health Organization, the U.S. Food and Drug Administration, and the U.K. Medicines and Healthcare Products Regulatory Agency recommend dengue vaccines should be given to persons living or visiting dengue-risk areas.

Novavax Inc. today announced the full results from the pediatric expansion of the Phase 3 PREVENT-19 clinical trial were published in the Journal of the American Medical Association Network Open on April 26, 2027.
The study's expansion evaluated the safety and effectiveness of Novavax's COVID-19 prototype vaccine (NVX-CoV2373) in adolescents aged 12 through 17 across the U.S.
Novavax's U.S. Food and Drug Administration-approved vaccine achieved its primary effectiveness and efficacy endpoint in the trial when the Delta variant was the predominant circulating SARS-CoV-2 coronavirus strain.
There was no increase in reactogenicity in younger (12 to <15 years old) adolescents compared to older (15 to <18 years old) adolescents.
Non-inferior neutralizing antibody responses compared to young adults in the main adult study were demonstrated, which was the critical regulatory endpoint for authorization.
And safety data showed the vaccine to be generally well-tolerated in the placebo-controlled portion of the study.
As of April 27, 2023, Novavax protein-based COVID-19 vaccines have been authorized in more than 30 markets worldwide, known as Nuvaxovid™, CovoVax™, and NVX-CoV2373.

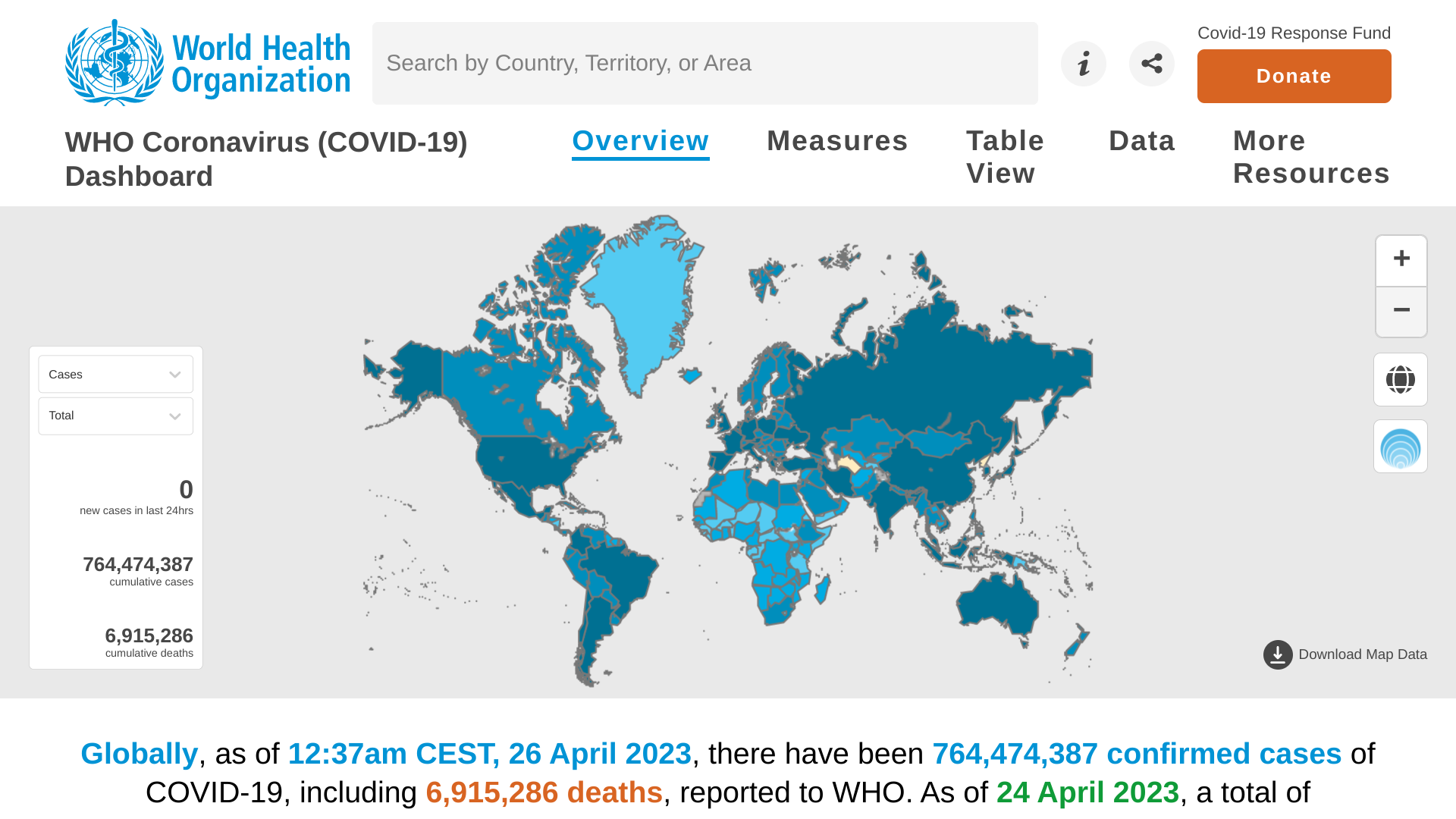
The World Health Organization (WHO) today published Edition #140 of its Weekly epidemiological update on the COVID-19 pandemic.
As of April 27, 2023, the WHO confirmed nearly 2.8 million new COVID-19 cases, and over 16,000 related fatalities were reported in the last 28 days.
This data indicates a decrease of 23% and 36%, respectively, compared to the previous period.
Contrary to the overall trend, increases in reported cases and deaths continued to be registered in the South-East Asia and Eastern Mediterranean regions and several individual countries elsewhere.
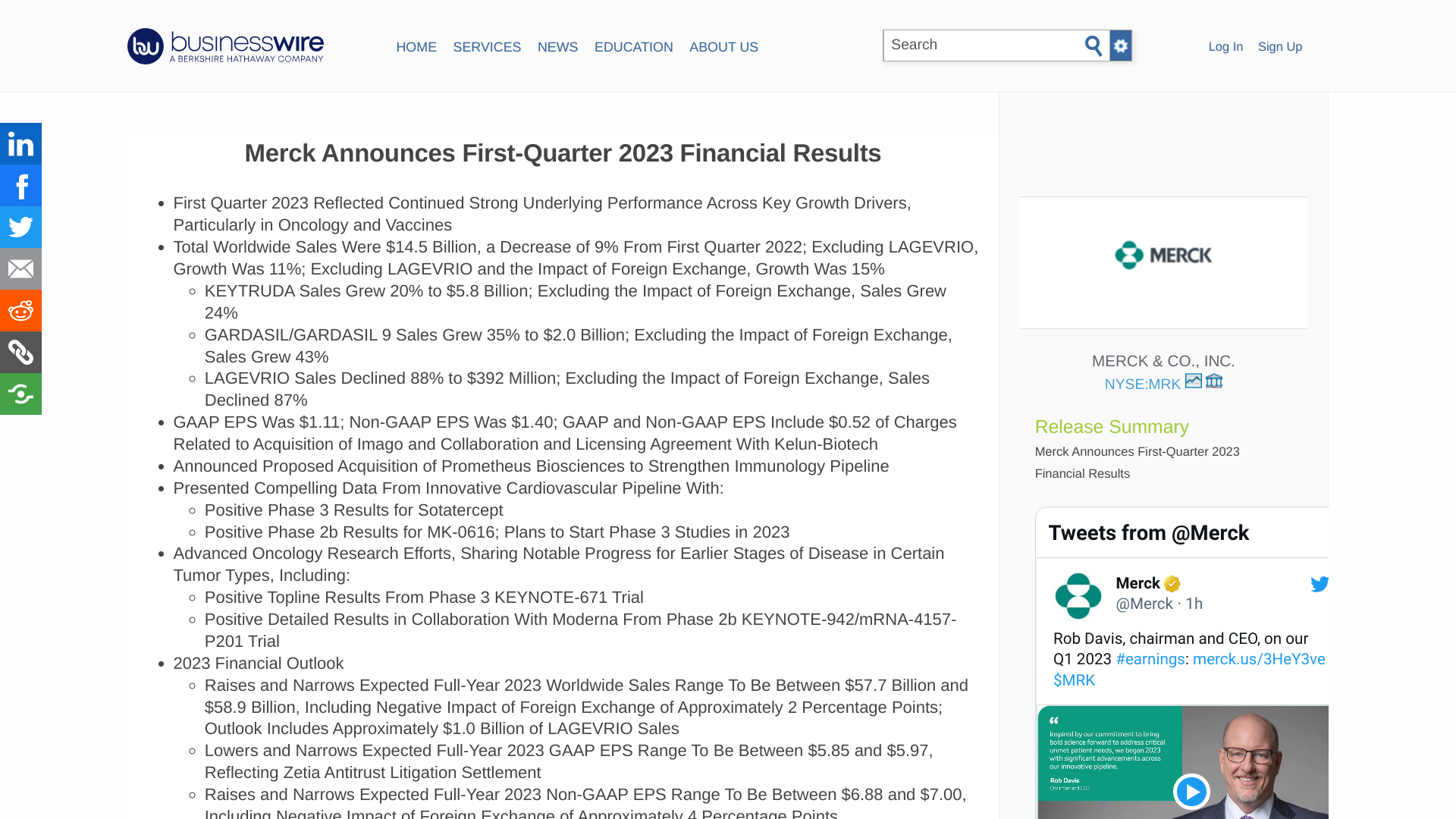
Merck today announced financial results for the first quarter of 2023. In addition, Merck stated it realized lower sales of the oral COVID-19 antiviral Lagevrio™ (Molnupiravir), which decreased 88% to $392 million.
This decrease was primarily attributable to sales in the U.S. and U.K. markets in the first quarter of 2022 that did not recur in the first quarter of 2023.
LAGEVRIO's decline was also attributable to lower sales in Japan and Australia.
LAGEVRIO is approved or authorized for use in more than 25 countries. It helps reduce how sick people become when diagnosed with COVID‑19.
Additional COVID-19 antiviral news is posted at Coronavirus Today.