Search API
InflaRx N.V. recently announced that Gohibic (vilobelimab), a first-in-class monoclonal anti-human complement factor C5a antibody, has been granted an Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving invasive mechanical ventilation, or extracorporeal membrane oxygenation.
The FDA issued EUA #118 on April 14, 2023.
InflaRx has a supply of Gohibic available and is working to ramp up production at its third-party manufacturer to roll out supply in the U.S. as soon as possible.
In a related press release, Prof. Niels C. Riedemann, CEO and Founder of InflaRx, said, "We are thrilled and very proud that the FDA has issued a EUA for vilobelimab to treat this very sick patient group, recognizing the lifesaving potential of this first-in-class drug."
According to the U.S. National Institutes of Health (NIH), monoclonal antibody (mAbs) products that target the SARS-CoV-2 coronavirus spike protein have been shown to have clinical benefits in limiting and treating infections that cause COVID-19. mAbs treatments block SARS-CoV-2 from entering cells in the human body.

Moderna, Inc. today announced financial results and provided business updates for the first quarter of 2023.
"We had a strong first quarter, with $1.9 billion in revenue, clearly indicating that we are on our way to deliver on the $5 billion of signed Advance Purchase Agreements for 2023," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.
"In addition, we are encouraged by the progress of new COVID-19 vaccine contracts in the U.S. for this fall with pharmacy chains, hospital networks, and multiple U.S. government agencies."
"Similar discussions are ongoing with Japan, the EU, and other key markets such as Australia, which recently ordered additional COVID-19 vaccines."
"..... we are fully preparing for potential commercial launches of two products in 2024, our RSV and flu vaccines."
Moderna's respiratory pipeline includes Phase 3 trials against RSV, influenza, and a next-generation COVID-19 candidate.
The mRNA pipeline includes four additional influenza vaccines with expanded antigens, vaccines against other respiratory pathogens (e.g., hMPV), and six combination vaccine programs.
Moderna's unedited press release is posted at this link.

The World Organization for Animal Health (WOAH) published its latest global update confirming highly pathogenic avian influenza (HAPI) outbreaks continue in various countries.
As of May 3, 2023, the current HPAI epidemic season continues, with 48 outbreaks reported in poultry and 33 in non-poultry birds over the three weeks covered by the report.
About 1.5 million poultry birds died or were culled worldwide.
These HAPI detections were mainly in Europe (36), the Americas, and Asia.
And the first occurrence of HPAI in non-poultry birds in the Republic of Gambia (Africa) at the end of March 2023 is noteworthy and shows that the HAPI disease is spreading to new areas.
Furthermore, based on the HPAI seasonal pattern, the number of outbreaks in animals (mammals) is expected to have passed the peak and decline.
However, the USDA's Animal and Plant Health Inspection Service reported six more H5N1 detections in mammals, bringing the total to 176 during this outbreak.
From a human risk perspective, official health authorities of the People's Rep. of China notified the World Health Organization on March 27, 2023, of one confirmed case of human infection with an avian influenza A(H3N8) virus.
This is the third reported case of human infection with an avian influenza A(H3N8) virus reported by China.
While the annual flu shot does not offer protection against HAPI viruses, there are approved bird flu vaccines and vaccine candidates in development.

The peer-review journal JAMA Network today published updated U.S. Preventive Services Task Force (USPSTF) tuberculosis (TB) screening and treatment guidelines.
The USPSTF estimated TB prevalence is about 5% or up to 13 million persons.
In the U.S., TB remains a significant preventable disease, including active tuberculosis, which may be infectious, and latent tuberculosis infection (LTBI), which is asymptomatic and not infectious but can later progress to active disease.
Announced on May 2, 2023, the USPSTF concluded with a moderate net benefit in preventing active TB by screening for LTBI in persons at increased risk for tuberculosis infection.
The precise prevalence rate of LTBI in the US is difficult to determine.
TB is spread through respiratory transmission.
Approximately 30% of persons exposed to Mycobacterium tuberculosis will develop LTBI, and if left untreated, about 5% to 10% of healthy, immunocompetent persons will progress to having active tuberculosis disease.
This recommendation replaces the 2016 USPSTF recommendation on LTBI screening.
In the U.S., various cities and states are confronting TB outbreaks in 2023.
The Centers for Disease Control and Prevention reported on March 23, 2023, TB cases increased by 5% or more in some cities, such as Houston, in 2022.
Unfortunately, this recommendation does not mention the 100-year-old BCG vaccine as an option to protect children from TB.
As of May 3, 2023, numerous countries offer various BCG vaccines to children to prevent TB.

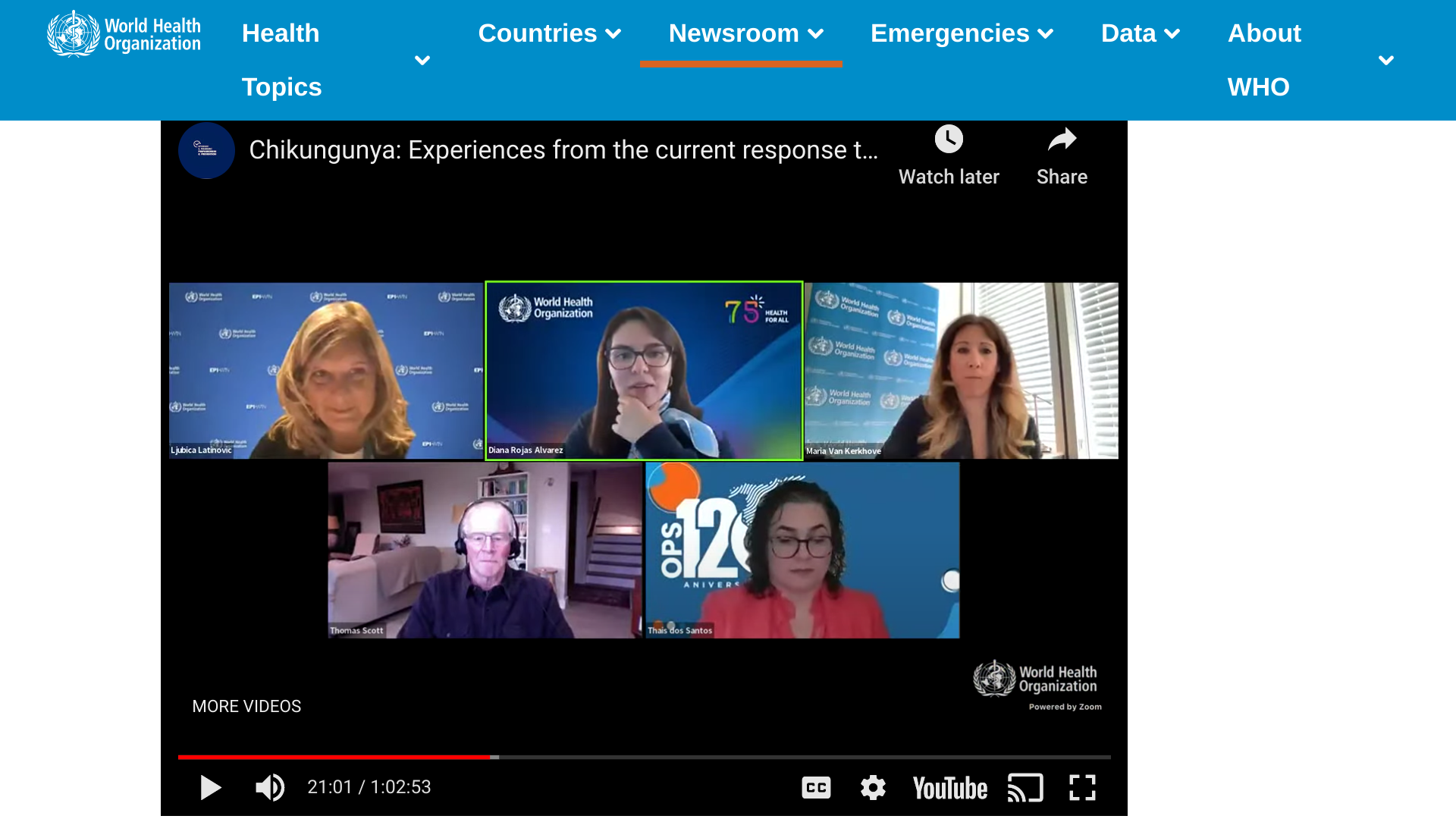
The World Health Organization (WHO) is hosting a free webinar today titled: Chikungunya: Experiences from the current response to outbreaks in the Americas.
Chikungunya is a viral disease transmitted to humans through the bites of Aedes mosquitoes infected with the chikungunya virus. It has been identified in nearly 115 countries in all the continents except Antarctica.
There are chikungunya vaccine candidates in development as of May 3, 2023. However, there are no approved vaccines.
On May 3, 2023, the speakers were:
- Dr. Sylvie Briand, Director, Epidemic and Pandemic Preparedness and Prevention (EPP), WHO
- Dr. Maria Van Kerkhove, Unit Head, Emerging Diseases and Zoonoses Unit, WHO
- Dr. Thomas Scott, Distinguished Professor of mosquito-transmitted disease ecology and epidemiology, University of California, USA
- Dr. Diana Rojas Alvarez, Technical Lead - Zika and Chikungunya, WHO
- Ms. Thais dos Santos, Advisor on Surveillance and Control of Arboviral Diseases, WHO PAHO
This session can be watched on YouTude.
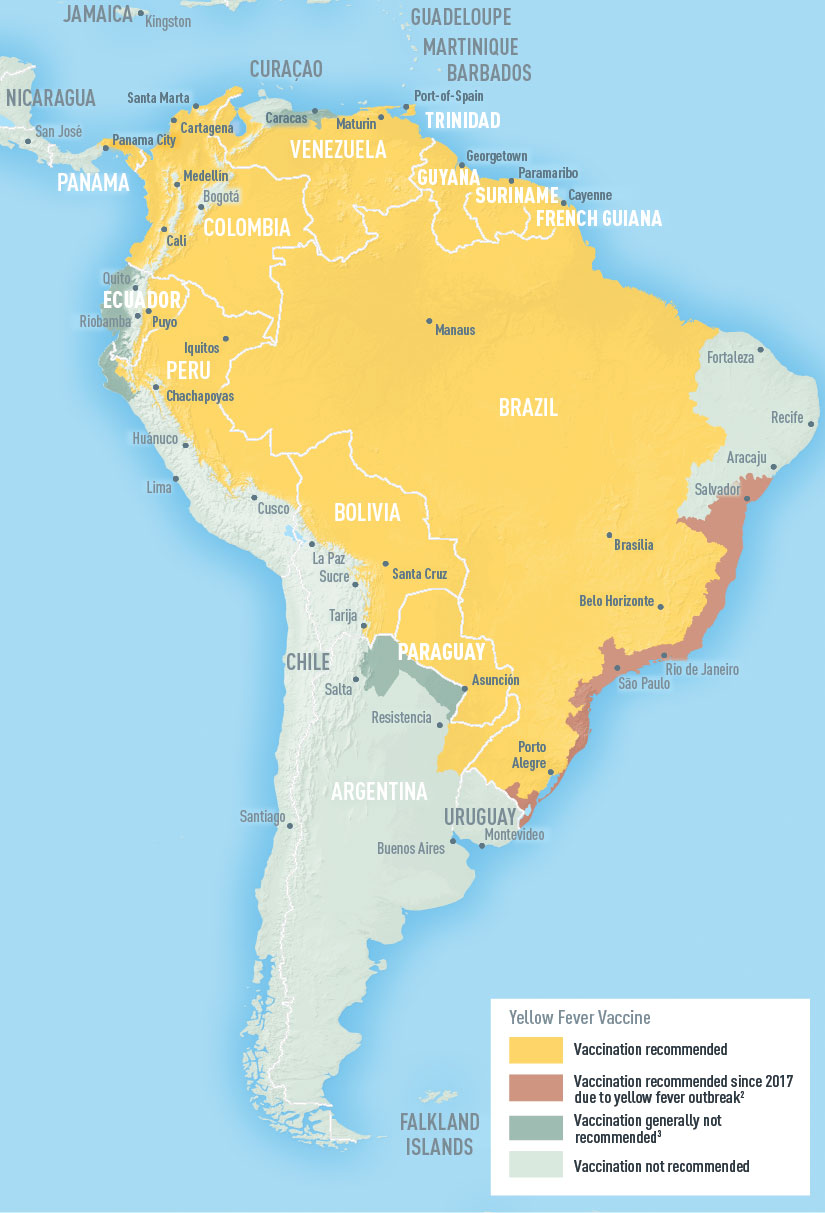
The Pan American Health Organization / World Health Organization (PAHO/WHO) latest Epidemiological Update highlights yellow fever hot spots in the Region of the Americas.
As of April 25, 2023, the PAHO/WHO reported that in 2023, human yellow fever cases have been recorded in Bolivia (two cases) and Brazil (three cases).
In 2022, confirmed cases of yellow fever were reported in three countries in the Americas: Bolivia (5 confirmed cases), Brazil (5 cases, including four deaths), and Peru (7 confirmed cases, including five deaths).
As of May 3, 2023, various countries require proof of yellow fever vaccination at airports upon arrival.
In the U.S., yellow fever vaccine (YF-VAX®) availability is limited to certified clinics and travel pharmacies.
Internationally, the Stamaril® is generally available in 2023.
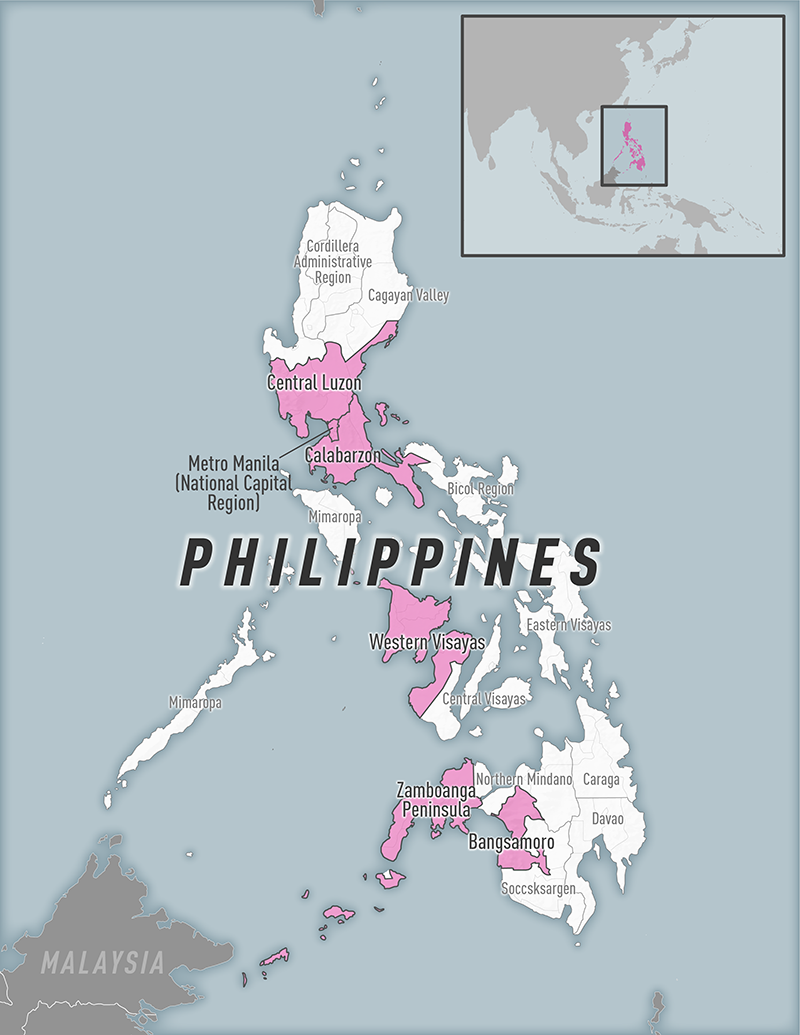
The U.S. Centers for Disease Control and Prevention (CDC) today announced it issued an Alert - Level 2, Practice Enhanced Precautions regarding the Republic of the Philippines reporting increased diphtheria cases in various regions.
As of May 1, 2023, the CDC says if you are traveling to an affected area, you should be up to date with your diphtheria vaccines.
And before visiting the Philippines, discuss the need for a booster dose with your healthcare professional.
Diphtheria is a severe infection caused by strains of Corynebacterium diphtheriae bacteria that make a toxin.
When the bacteria enter the respiratory system, they can cause sore throat, mild fever, and swollen glands in the neck. In addition, the bacteria make a toxin that kills healthy tissues in the respiratory system and can make it difficult to breathe and swallow.
For some people, respiratory diphtheria can lead to death. Even with treatment, about 1 in 10 patients with respiratory diphtheria die.
Without treatment, up to half of patients can die from the disease, says the CDC.
Additionally, the CDC included the Philippines in a recent dengue outbreak alert.