Search API
The World Health Organization (WHO) today announced that the Fifth Meeting of the International Health Regulations Emergency Committee on the Multi-Country Outbreak of Mpox decided to end the public health emergency of international concern (PHEIC).
As of May 11, 2023, data sources indicate about 87,000 Mpox cases have been confirmed globally, with 30,395 cases and 42 related fatalities in the U.S. since early May 2022.
The WHO committee stated having considered the significant decline in the global spread of mpox and the gains achieved in the control of the outbreak in many countries; the Committee advised that the event requires a transition from a PHEIC to a robust, proactive and sustainable mpox response and control program.
The Committee emphasized the need for long-term attention and support, including financial aid, particularly for countries where Mpox occurs regularly, and advised that Standing Recommendations would now be a more appropriate tool to manage the immediate, short, and long-term public health risks posed by Mpox.
In the U.S., an initial Mpox outbreak was declared in August 2022. On October 27, 2022, the Mpox public health emergency in San Francisco ended, followed by New York City in November 2022.
And on January 31, 2023, the U.S. HHS did not renew the Mpox public health emergency declaration.
However, recent Mpox outbreaks have been reported in Chicago.
Furthermore, the U.S. Food and Drug Administration previously approved vaccines can prevent certain sexually transmitted diseases such as Mpox.
And the oral TPOXX® (Tecovirimat) treatment remains available in the U.S.

The U.S. Centers for Disease Control and Prevention (CDC) has scheduled for today at 2 pm ET a Clinician Outreach and Communication Activity (COCA) call with updated recommendations for COVID-19 vaccine use.
During this COCA Call on May 11, 2023, presenters will discuss updated COVID-19 vaccine recommendations by age group and for those with immunocompromise.
Sara Oliver, MD, MSPH, and Evelyn Twentyman, MD, MPH, will also highlight optional COVID-19 vaccine doses for specific populations.
With a simplified schedule, the CDC recommends that everyone stay up to date with recommended COVID-19 vaccines to better protect themselves from severe illness.
Most people in the United States have not yet received an updated (bivalent) mRNA COVID-19 vaccine, with less than 17% uptake.
The Webinar Link: https://www.zoomgov.com/j/1619378091; ID: 161 937 8091; Passcode: 499465.
Takeda today announced its dengue vaccine QDENGA® had received several approvals, including one by Brazil's National Health Surveillance Agency in March 2023 for use in individuals between 4 years and 60 years to protect against all four serotypes.
Brazil's approval marks the first approval of QDENGA in Latin America, where dengue is endemic in various countries.
Recently, Costa Rica confirmed a unique dengue outbreak.
QDENGA is the only dengue vaccine approved for use regardless of previous exposure and without needing pre-vaccination testing.
Takeda's press release on May 11, 2023, confirmed it continues progressing with additional regulatory filings, such as the U.S.
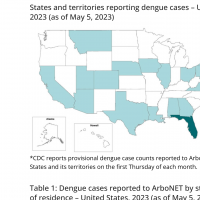
The U.S. CDC published provisional data as of May 5, 2023, indicating the U.S. States, led by Florida, have reported 98 dengue cases in 2023, and U.S. Territories have confirmed 238 dengue cases this year.
The Kingdom of Lesotho recently confirmed a measles outbreak in the capital city of Maseru, which has a population of over 300,000.
In response, through the Maseru District Health Management Team, the Ministry of Health launched a measles vaccination campaign in mid-April 2023.
Measles is a severe disease that can be prevented with safe and effective vaccines.
Lesotho is located in the country of South Africa, which has been reporting measles outbreaks in 2023.
South Africa's National Institute for Communicable Diseases reported in April the percentage of samples testing positive for measles remained at 27%.
Unfortunately, the Mountain Kingdom's 2022 measles vaccination campaign turnout was about 33% in 2022.
Before 2019, Lesotho had gone about ten years without a measles outbreak.
Measles outbreak news and alerts are posted by Vax-Before-Travel.

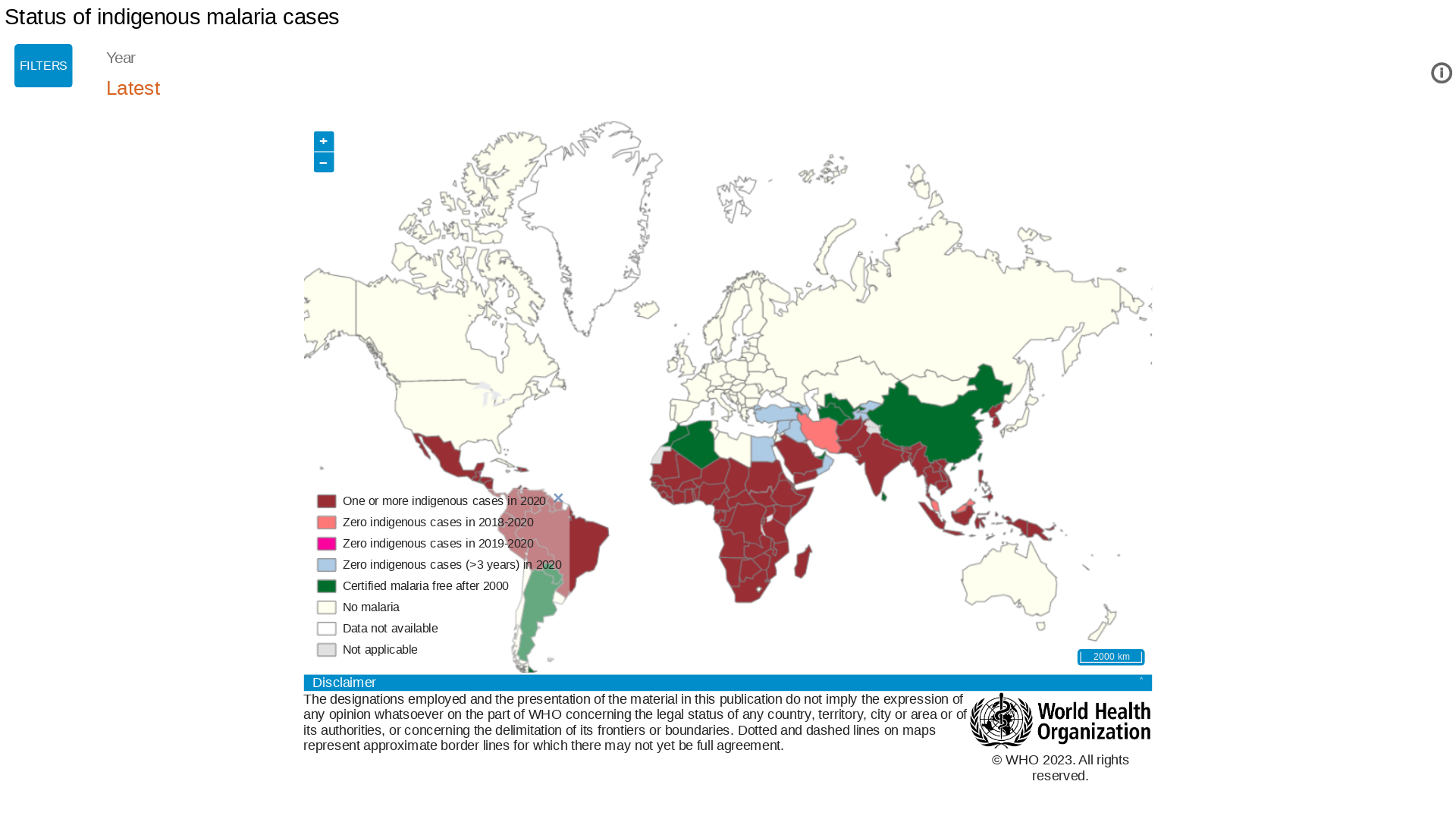
Vakzine Projekt Management GmbH (VPM) today announced the successful licensing of the novel R21/Matrix-MTM Malaria Vaccine by the Ghana Food and Drugs Authority.
On May 10, 2023, VPM confirmed in a press release that the R21/Matrix-M™ vaccine has emerged as the most effective vaccine against malaria.
This innovative malaria vaccine was initially developed by the lab research team of Adrian Hill, Director of the University of Oxford's Jenner Institute.
Based on available clinical data in 2023, the R21/Matrix-M™ vaccine's efficacy is greater than 75%, far above the effectiveness of the other approved malaria vaccine, Mosquirix™ (RTS,S/AS01).
Notably, the manufacturing capacity of 200 million doses/year by SII will increase the current supply capacities by >20-fold globally.
Malaria is one of the leading causes of pediatric morbidity and mortality in sub-Saharan Africa. And that children under five account for approximately 80% of all malaria-related fatalities, says the WHO Africa.
Four African countries accounted for just over half of all malaria deaths worldwide: Nigeria (31.3%), the Democratic Republic of the Congo (12.6%), the United Republic of Tanzania (4.1%), and Niger (3.9%).
And in the Northern Hemisphere, Costa Rica recently reported 105 positive malaria cases.
Malaria outbreak news is posted by Vax-Before-Travel.
Sinovac Biotech Ltd. today announced it would provide its CoronaVac® vaccine to self-paying groups in Hong Kong and confirmed a donation plan.
Additionally, the company will collaborate with local charity groups to provide donations, giving more children in Hong Kong access to free its inactivated original strains COVID-19 vaccine.
On March 31, 2023, the Government of the Hong Kong Special Administrative Region announced that beginning April 20, 2023, high-risk groups would be able to receive free doses of COVID-19 vaccines.
Whereas low-risk groups, including children, can only receive their vaccines via the private market through self-pay.
"SINOVAC's ongoing fight against COVID-19 is based on our mission to 'supply vaccines to eliminate human diseases,'" said Yin Weidong, Chairman, President, and CEO of SINOVAC, in a press release on May 10, 2023.
"From developing the COVID-19 vaccine to obtaining market approvals, from the first CoronaVac® shipment landing in Hong Kong 800 days ago to the rollout of a massive public vaccination program, SINOVAC and Hong Kong have taken steps together to emerge from the shadows of the pandemic."
CoronaVac® is the first COVID-19 vaccine to be used in children from as young as three years old, under the World Health Organization's Emergency Use List.
As of March 2023, CoronaVac® has been authorized for use in more than 60 countries, regions, and international organizations. But not in the U.S.
The cumulative global vaccine supply exceeds 2.9 billion doses.