Search API
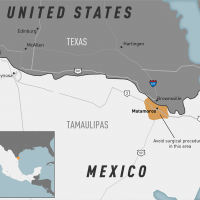
During the U.S. Centers for Disease Control and Prevention COCA Call today, experts confirmed new mpox cases have occurred in some vaccinated men in Chicago, Illinois.
These mpox patients were vaccinated less than one year ago.
On side #46, the COCA call presented from March 18 through May 15, 2023:
- 21 men reported mpox infections to the Chicago Dept. of Public Health,
- 17 cases (of 21 with information) were vaccinated,
- 11 with two doses of the JYNNEOS® vaccine, 5 with one dose, and 1 with the smallpox vaccine ACAM2000,
- 5 had well-controlled HIV,
- And none were hospitalized.
'It is important that clinicians quickly identify cases to limit a possible mpox resurgence this summer in the United States,' wrote these CDC experts.
A replay of the COCA Call on May 18, 2023, will be accessible on this CDC webpage https://emergency.cdc.gov/coca/calls/2023/callinfo_051823.asp
The U.S. Food and Drug Administration (FDA) today published the Briefing Document for the Vaccines and Related Biological Products Advisory Committee (VRBPAC) review of ABRYSVO™, a respiratory syncytial virus (RSV) vaccine.
Pfizer Inc.'s ABRYSVO is a bivalent vaccine candidate comprised of two preF proteins selected to optimize protection against RSV A and B.
This digital meeting is scheduled for May 18, 2023, and starts at 8:30 AM ET and is open to the public.
The VRBPAC provides independent expert advice to the FDA on broad scientific topics or certain products to help the agency make sound decisions based on the available science.
GSK's AREXVY™ RSV OA single-dose RSV vaccine was previously approved by the FDA for seniors.
Furthermore, there are several other RSV vaccine candidates conducting late-stage studies.
Update May 18, 2023 - EXECUTIVE SUMMARY - This document summarizes the favorable benefit-risk profile for Pfizer’s RSVpreF (Abrysvo), a bivalent respiratory syncytial virus (RSV) stabilized prefusion F subunit vaccine (RSVpreF) for the proposed indication for prevention of lower respiratory tract disease and severe lower respiratory tract disease caused by RSV in infants from birth through 6 months of age, by active immunization of pregnant individuals.

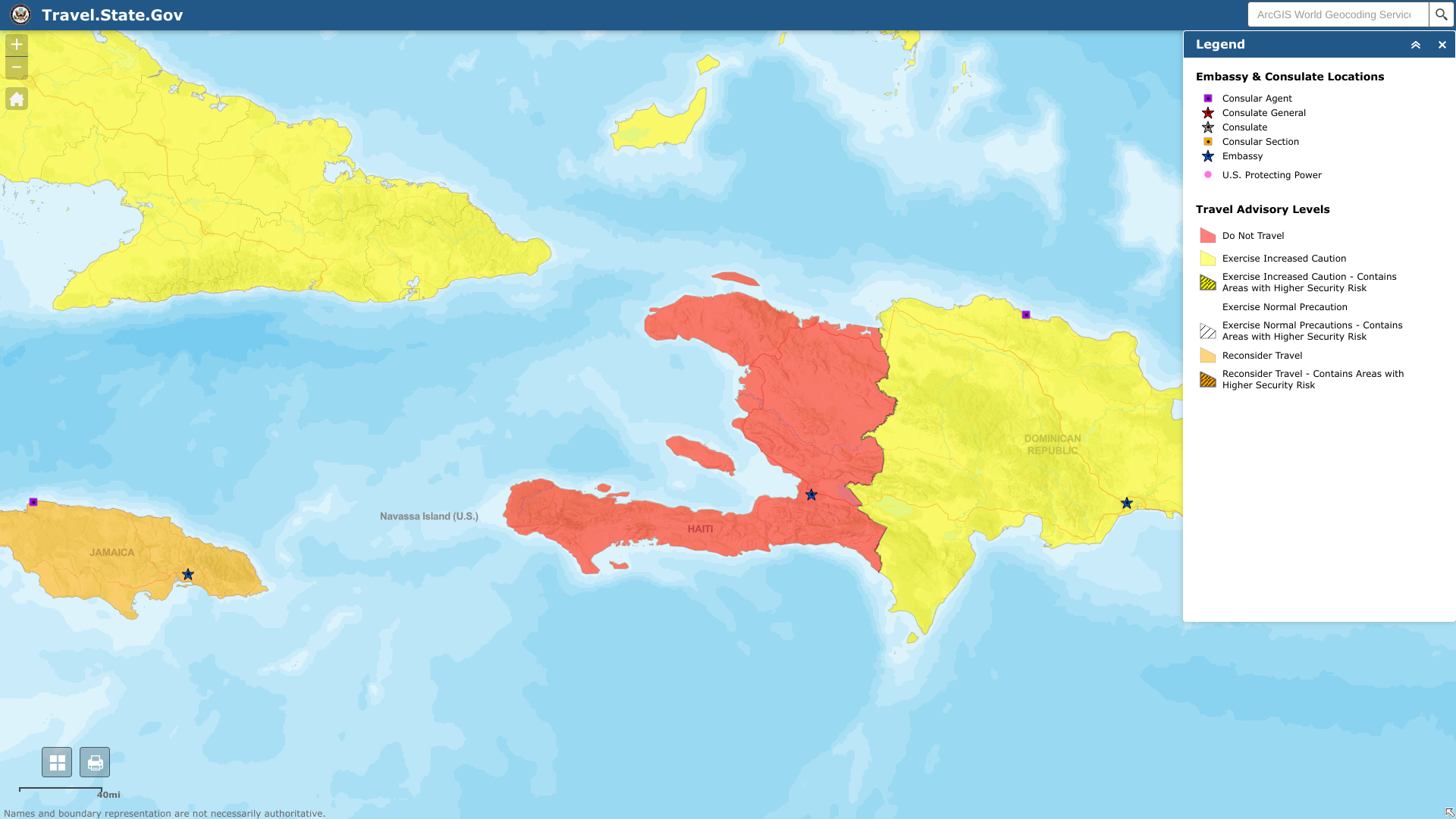
The U.S. Department of Stated today reissued its Level 4: Do Not Travel for the Republic of Haiti due to civil unrest.
Announced on May 17, 2023, the Department of States says U.S. citizens in Haiti should consider departing Haiti by commercial or other privately available transportation options in light of the current security situation and infrastructure challenges.
The U.S. Embassy in Port-au-Prince suspended employee travel to Cap Haitien from May 17-21, 2023.
And U.S. citizens wishing to depart Port-au-Prince should monitor local news and only do so when considered safe.
Furthermore, U.S. government personnel are discouraged from walking in Port-au-Prince.
And only family members over the age of 18 are permitted to accompany U.S. government employees assigned to the U.S. Embassy in Port-au-Prince.
From a health perspective, the Haitian Ministry of Health and Population recently confirmed an ongoing cholera outbreak.
Additionally, the U.S. CDC recommends various travel vaccinations, such as typhoid and yellow fever.
Other Disease Hot Spots are posted by Vax-Before-Travel.
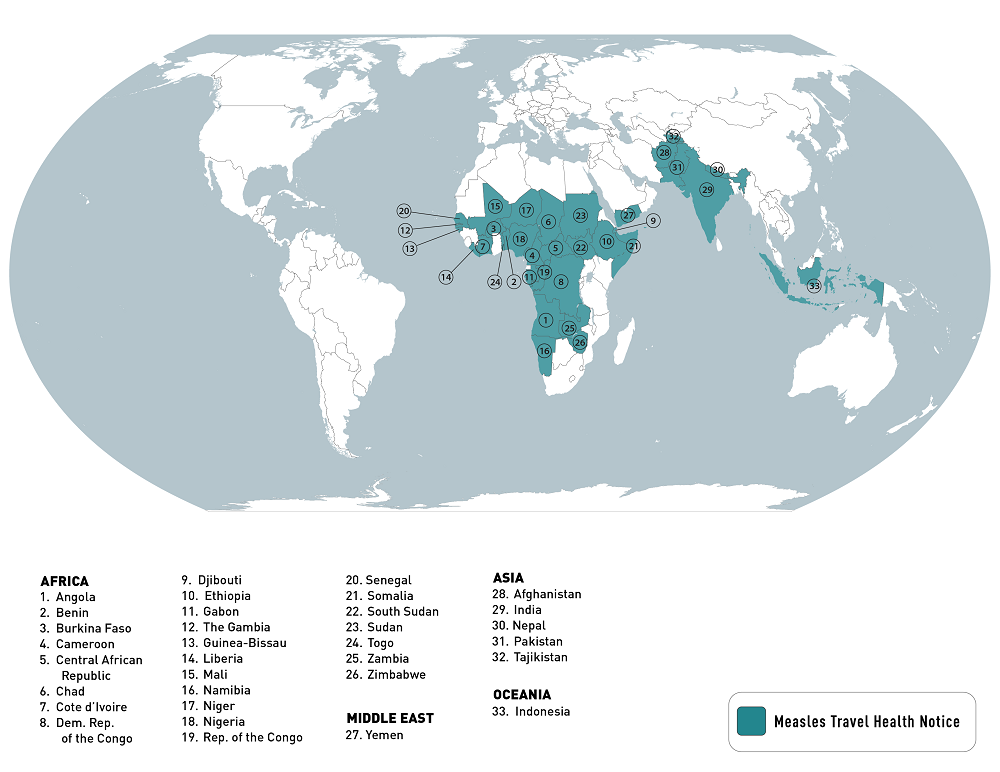
The U.S. Centers for Disease Control and Prevention (CDC) today reissued its Watch - Level 1, Practice Usual Precautions notice regarding the ongoing worldwide measles outbreak.
As of May 16, 2023, the CDC has compiled an extensive list of countries reporting measles outbreaks in 2023.
This list is led by India, with about 68,000 measles cases.
Measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes.
The measles virus can live for up to two hours in airspace after an infected person leaves an area.
Furthermore, people can spread measles up to four days before and four days after a rash.
While there have only been ten measles cases in the U.S. this year, this virus remains a risk to anyone under-vaccinated.
The CDC says all international travelers, including infants and preschool-aged children, should be protected against measles before traveling abroad.
Various measles vaccines are offered in the U.S. at clinics and community pharmacies.
A clinical trial of an experimental universal influenza vaccine candidate developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC) has begun enrolling volunteers.
This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.
“A universal influenza vaccine would be a major public health achievement and could eliminate the need for both annual development of seasonal influenza vaccines, as well as the need for patients to get a flu shot each year,” said Acting NIAID Director Hugh Auchincloss, M.D., in a press release on May 15, 2023.
“Moreover, some strains of the influenza virus have significant pandemic potential. A universal flu vaccine could serve as an important line of defense against the spread of a future flu pandemic.”
A similar vaccine developed by researchers at NIAID’s VRC has already shown positive results in early clinical trials.
Both vaccines use a specific portion of a flu protein, hemagglutinin (HA), to induce a broad immune response against influenza.
While one portion of the HA protein, known as the head, tends to change as the flu virus spreads and evolves, a more stable portion, known as the stem, evolves very slowly and is very similar across many different types of the flu virus.
Researchers hope to induce long-term immunity against a broad range of flu viruses by using the HA stem as the basis for a vaccine.
Unlike the VRC’s earlier vaccine, the H1ssF-3928 mRNA-LNP vaccine candidate uses an mRNA platform.
By developing and testing various platforms for a universal flu vaccine, researchers are more likely to find one that is safe and provides strong and broad immunity against various strains.
Additional flu shot and influenza vaccine development news is posted at Precision Vaccinations.

YS Biopharma Co., Ltd. today announced its PIKA Rabies Vaccine candidate received Phase 3 clinical trial approval from the Drug Regulatory Authority of Pakistan.
The PIKA Rabies Vaccine has the potential to become the first accelerated three-visit one-week regimen, superior to the currently available vaccine with a five-visit one-month or three-visit three-week regimen.
Dr. Zenaida Mojares, Chief Medical Officer of YS Biopharma, commented in a press release on May 16, 2023, "Our progress enables us to advance towards our mission of providing innovative and efficacious vaccines in the fight against a vaccine-preventable rabies disease, with almost 100% case fatality rate."
Rabies is a zoonotic infection that is a vaccine-preventable viral disease. Unfortunately, almost 60,000 people worldwide die from rabies each year.
The number of human rabies deaths in the United States has been steadily declining since the 1970s thanks to animal control (bats and dogs) and vaccination programs, says the U.S. CDC.
Up to 95% of human deaths occur in Africa and Asia, where dog rabies is poorly controlled, says the WHO.
The Company has completed Phase 1 and 2 clinical trials of its PIKA Rabies Vaccine in Singapore. Another Phase 1 trial was also conducted in China. All three trials have shown that the PIKA rabies vaccine is safe, tolerable, and immunogenic.
In the U.S., various rabies vaccines are available at clinics and community pharmacies.
Note: Starting February 1, 2023, the temporary suspension for dogs entering the U.S. from high-risk countries for dog rabies was extended. This includes dogs arriving from countries without a high risk of rabies if the dogs have been in a high-risk country in the past six months.
Emergent BioSolutions today announced it had completed the sale of its travel health business to Bavarian Nordic and may receive up to $380 million in potential future payments.
Bavarian Nordic acquired the rights to Vivotif®, the licensed typhoid vaccine, and Vaxchora®, the licensed cholera vaccine, and the development-stage chikungunya vaccine candidate CHIKV VLP.
These travel-related vaccines are part of an estimated international market growth rate (9.9%) thru 2028. This data indicates millions of travelers are under-vaccinated before visiting disease-endemic countries.
Bavarian Nordic also acquired manufacturing facilities in Bern, Switzerland, and development facilities in San Diego, California.
"This deal achieves two significant outcomes key to our mission and future success," said Robert G. Kramer, Emergent president, and chief executive officer, in a press release on May 15, 2023.
Other market research reports indicate an uplift in international travel is coming.
Such as the Expedia Group's Traveler Insights reveals traveler searches increasing globally by 25% in Q1 2023, which means travelers are looking toward mid-year getaways.