Search API
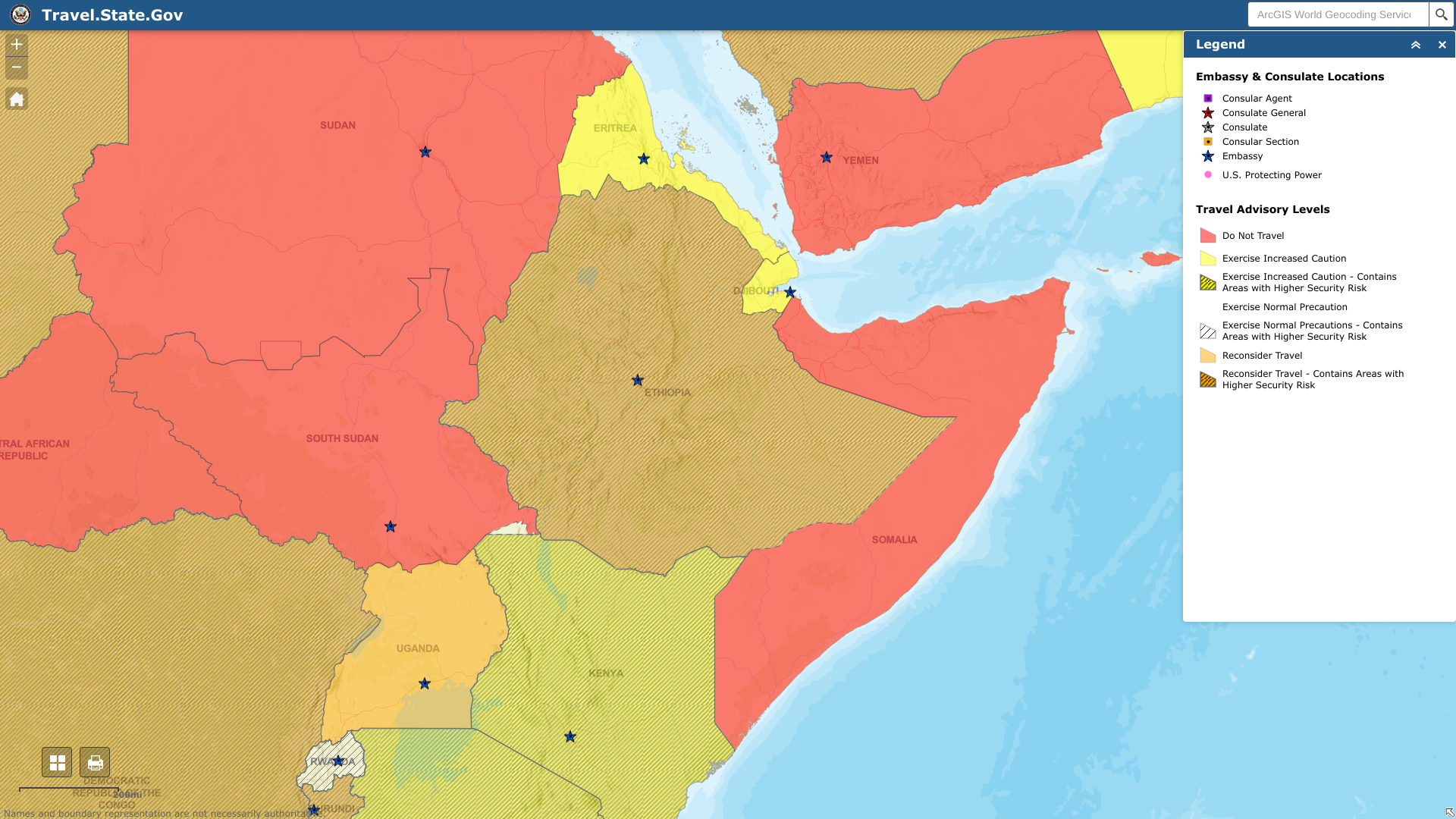
The U.S. Department of State recently reissued its Level 3 travel advisory for the Federal Democratic Republic of Ethiopia with updates to security information.
As of May 19, 2023, the U.S. says to reconsider travel to Ethiopia in cities and border areas due to sporadic civil unrest, crime, and communications disruptions.
U.S. officials have limited ability to provide services to U.S. citizens outside of Addis Ababa and any U.S. citizen detained by Ethiopian authorities.
And the government of Ethiopia has previously restricted or shut down internet, cellular data, and phone services before, during, and after civil unrest. These restrictions impede the U.S.
Please contact the Embassy’s American Citizen Services Unit at [email protected] for further assistance, and enroll in STEP to be located during emergencies.
When visiting Ethiopia, Do Not Travel To the following areas:
- Tigray Region and the border with Eritrea.
- Afar-Tigray border areas.
- Amhara Region.
- Gambella and Benishangul Gumuz Regions.
- Oromia Region.
- Southern Nations and National People Region.
- The border area with Somalia.
- Border areas with Sudan and South Sudan.
- Border areas with Kenya.
From a health perspective, the U.S. CDC suggests visitors ensure they are protected against measles and polio.
The Insight Partners today published its latest research report on "Influenza Vaccines Market Size Report, Share, Trends, Growth, Demand & Forecast to 2028, which estimated the CAGR of 7.4% from 2022 to 2028.
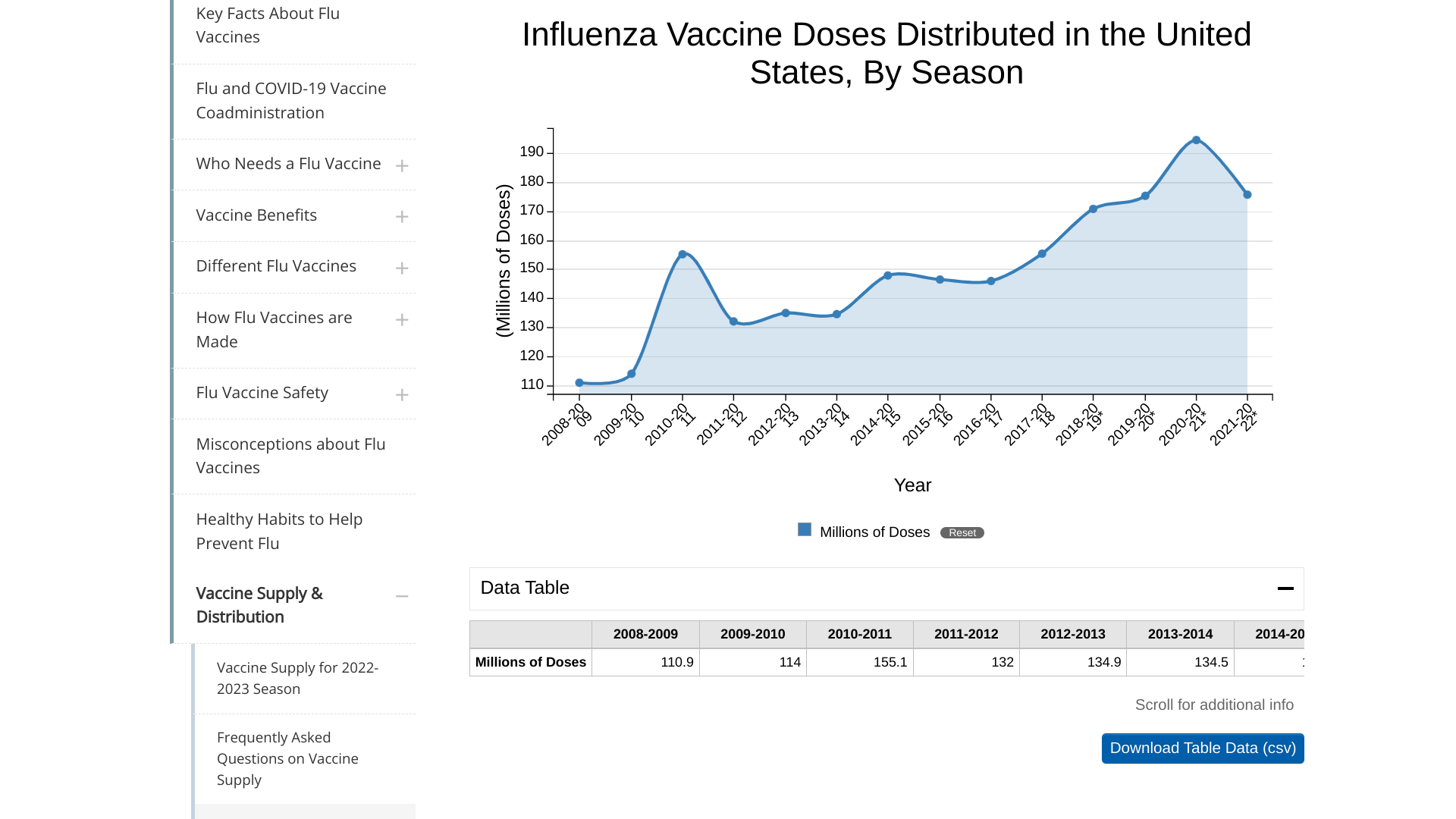
During the current flu season, the Centers for Disease Control and Prevention (CDC) reported as of March 4, 2023, about 173.37 million influenza vaccines had been distributed in the U.S.
This amount resembles the 2021-2022 flu season, when 174.9 million doses were distributed.
Additional flu shot news is posted by Precision Vaccinations.
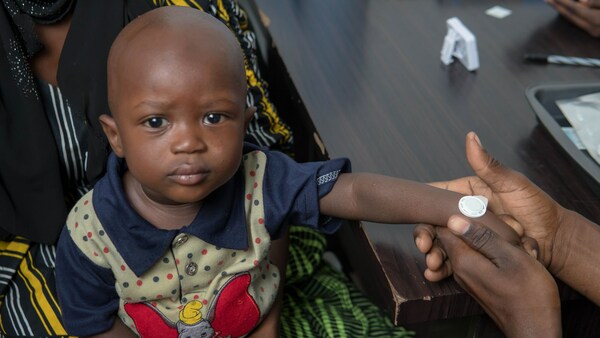
Micron Biomedical recently announced positive Phase 1/2 data from the first-ever clinical trial of microarray technology in children, including infants as young as nine months old.
The study evaluated the safety, immunogenicity, and acceptability of the leading commercially available measles-rubella (MR) vaccine administered via microarray.
Vaccination by microarray was found safe and well tolerated with no allergic reactions or related serious adverse events.
Day-42 immunogenicity showed seroprotection rates for MR in all cohorts for both the microarray (93.2% - 100%) and SC injection (89.8% - 100%) groups.
And in infants who were MR-vaccine naïve at the start of the trial, seroconversion rates were high and similar for both the microarray (92.9% -100%) and SC injection groups (89.7%-100%).
Over 90% of the parents of toddlers and infants enrolled in the trial, which took part in an acceptability survey, said that the microarray technology would be better than SC injection to give vaccines to children.
"Supporting innovations in vaccine delivery is critical to addressing ongoing health inequities," said James Goodson, Senior Scientist and Epidemiologist in the Global Immunization Division at the Centers for Disease Control and Prevention and co-investigator for the study, in a press release on May 17, 2023.
The technology significantly simplifies the transport, storage, and administration of vaccines traditionally delivered via injection and eliminates sharps waste.
The U.S. CDC Advisory Committee on Immunization Practices (ACIP) confirmed it had scheduled a vaccine review meeting open to the public for June 21-23, 2023.
Conducted at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, this meeting's purpose is to review scientific data and vote on vaccines and candidate recommendations for Respiratory Syncytial Virus vaccines; Recommendations for adult Polio vaccinations; Flu shots for the 2023-2024 season, Chikungunya, COVID-19, Dengue, Meningococcal, and Mpox vaccines.
This meeting's agenda will be led by Dr. Grace Lee, the ACIP Chair.
ACIP recommendations are public health guidance for the safe use of vaccines and related biological products.
The non-binding recommendations include the age(s) when the vaccine should be given, the number of doses needed, the amount of time between doses, and precautions and contraindications.

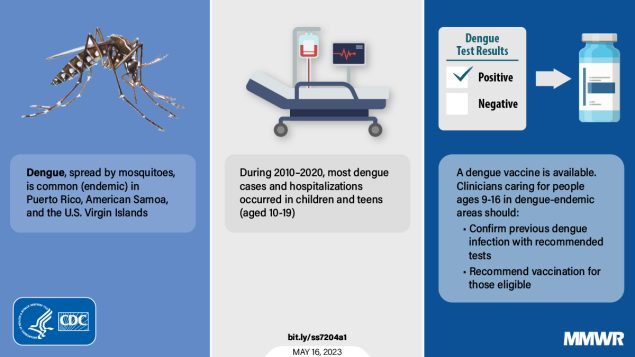
Dengue is one of the most common vectorborne flaviviral infections globally, with frequent outbreaks in the tropical regions of the United States, says the U.S. Centers for Disease Control and Prevention (CDC).
The CDC confirmed on May 19, 2023, U.S. territories experienced a high prevalence of dengue during 2010–2020, a total of 30,903 dengue cases were reported from four U.S. territories.
Puerto Rico reported the highest number of dengue cases (29,862).
And in Puerto Rico and USVI, approximately 2% of reported cases were categorized as severe dengue.
The Florida Health Department reported on May 13, 2023, there had been 68 travel-associated dengue cases and two locally acquired dengue cases confirmed as of week #17 in 2023. And Miami-Dade County remains under a mosquito-borne illness alert.
Floria reported 903 travel-associated and 68 locally-acquired dengue cases, the most noted in the U.S. in 2022.
Dengue is a vaccine-preventable disease, with two vaccines in use globally.
In addition, travel disease hot-spot news is posted by Vax-Before-Travel.
Pfizer Inc. today announced that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted that the available data support the efficacy and safety of its unadjuvanted bivalent respiratory syncytial virus (RSV) prefusion F vaccine candidate RSVpreF or PF-06928316 (ABRYSVO™).
The vaccine candidate is under FDA review for preventing medically attended lower respiratory tract disease (MA-LRTD) and severe MA-LRTD caused by RSV in infants from birth up to six months of age by active immunization of pregnant women.
The VRBPAC voted 14 to 0 on effectiveness and 10 to 4 on safety.
“We are encouraged by the outcome of today’s VRBPAC meeting as it is a critical step forward in the scientific community’s long-sought-after goal to help prevent RSV disease in infants during their most vulnerable first six months of life,” said Annaliesa Anderson, Ph.D., Senior Vice President and Chief Scientific Officer, Vaccine Research and Development, Pfizer, in a related press release.
Additional RSV vaccine and monoclonal antibody news are posted by Precision Vaccinations.

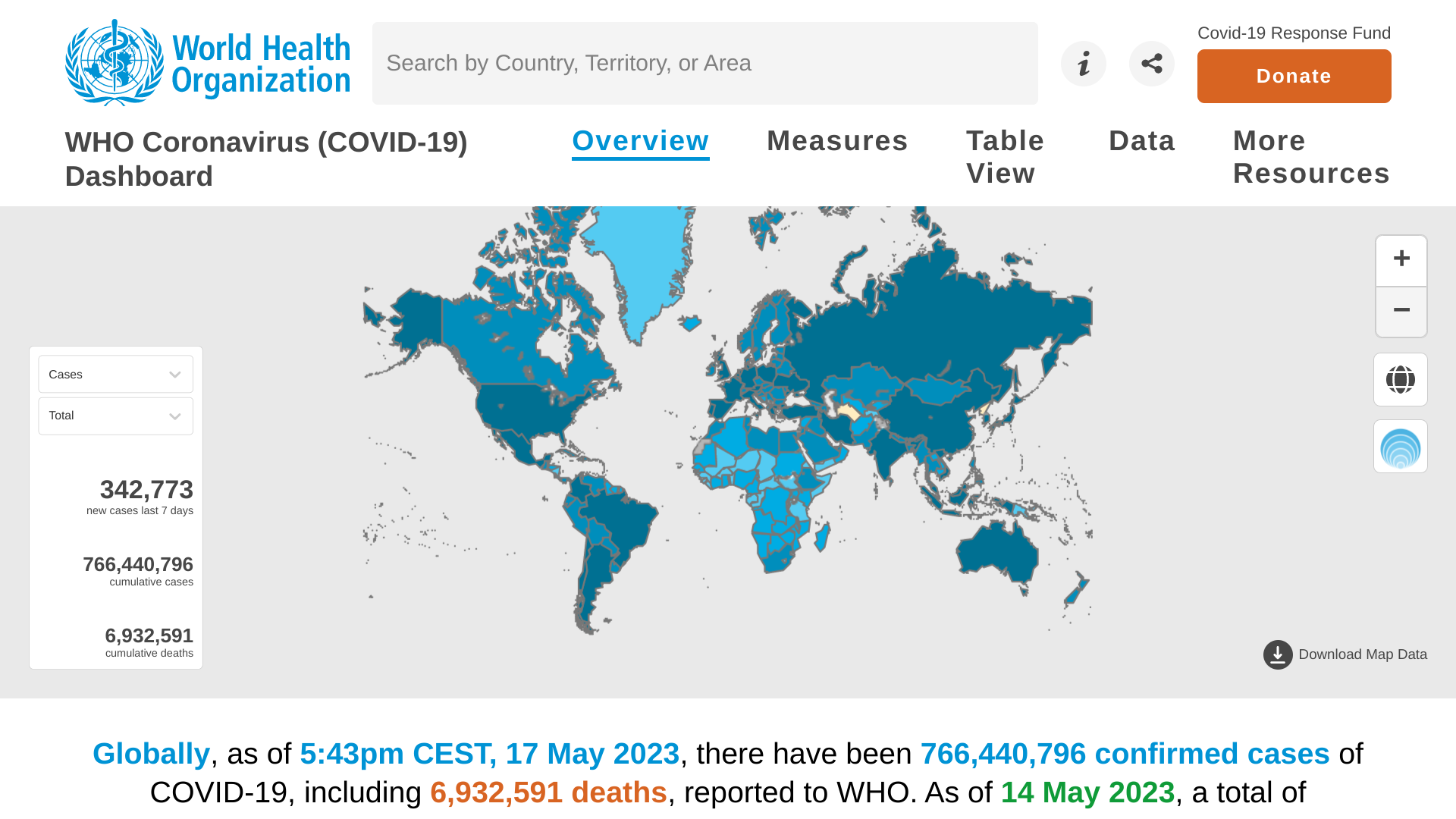
The World Health Organization (WHO) today announced nearly 2.6 million new COVID-19 cases, and over 17,000 deaths were reported in the last 28 days, a decrease of 14% and 26%, respectively, compared to the previous 28 days.
Weekly epidemiological update #143 was published on May 18, 2023, and stated the COVID-19 situation is mixed at regional levels, with increases in reported cases in South-East Asia and Western Pacific regions and increases in deaths in South-East Asia.
At the country level, the highest numbers of new 28-day cases were reported from the Republic of Korea (418, 960 new cases; +46%), the United States of America (-34%), Japan (+15%), India (+32%), and Brazil (-28%).
The U.K. Health Security Agency (UKHSA) recently detected influenza A (H5) virus in two poultry workers who have recently worked on an infected poultry farm in England.
Neither person has experienced any avian influenza (bird flu) symptoms, and both have since tested negative.
Professor Susan Hopkins, Chief Medical Advisor at UKHSA, stated in a press release on May 16, 2023, "Current evidence suggests that the avian influenza viruses we're seeing circulating in birds around the world do not spread easily to people."
"However, we know already that the virus can spread to people following close contact with infected birds, and this is why, through screening programs like this one, we are monitoring people who have been exposed to learn more about this risk."
"Globally, there is no evidence of the spread of this strain from person to person, but we know that viruses evolve all the time, and we remain vigilant for any evidence of changing risk to the population."
"It remains critical that people avoid touching sick or dead birds."
In the U.S., one bird flu vaccine is approved by the U.S. FDA.
Precision Vaccinations posts updated news on the global avian influenza outbreak in birds, mammals, and humans.

The World Health Organization (CDC) Technical Advisory Group for COVID-19 Vaccine Composition today announced its advice on the composition of future formulations of COVID-19 vaccines.
The objective of an update to COVID-19 vaccine antigen composition is to enhance vaccine-induced immune responses.
Updating the vaccine composition considers the evolution of the SARS-CoV-2 beta coronavirus variants and aims to improve protection against symptomatic disease.
The Group suggests that future formulations of COVID-19 vaccines use newer variants in their composition, i.e., XBB.1 descendant lineages.
As of May 2023, the XBB.1 descendent lineages currently predominate globally (i.e., XBB.1.5, XBB.1.16, XBB.1.9).
Furthermore, estimates of vaccine efficacy (VE) against currently circulating SARS-CoV-2 variants, including XBB.1 descendent lineages, are very limited in terms of the number of studies, vaccine products evaluated, and populations assessed; some studies show similar VE against BA.5 descendent and XBB.1 descendent lineages, while others suggest reduced VE during periods of the predominance of XBB.1 descendent lineages.
Additionally, the TAG-CO-VAC continues to encourage the further development of vaccines that enhance mucosal immunity because they may improve protection against infection and reduce transmission of SARS-CoV-2, in alignment with the WHO Global COVID-19 Vaccination Strategy, published in July 2022.
On May 18, 2023, the WHO stated the current COVID-19 vaccines continue to be highly protective against severe disease and death.
And the WHO strongly encourages the use of available authorized COVID-19 vaccines, which include the index SARS-CoV-2 virus, according to recommendations from the Strategic Advisory Group of Experts on Immunization, updated in March 2023.

