Search API
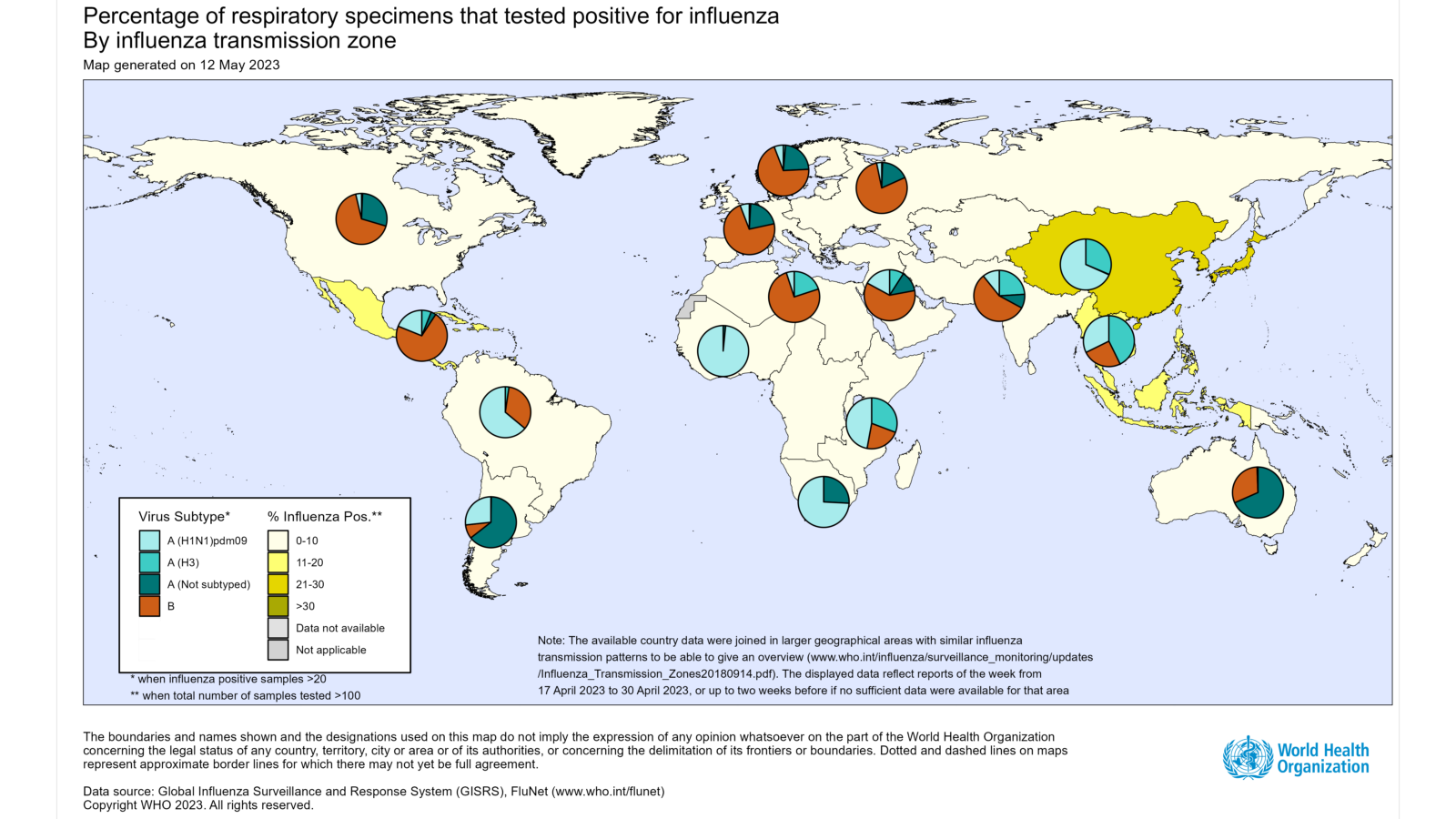
The World Health Organization (WHO) published Influenza Update N° 445 on May 15, 2023, stating influenza detections decreased further due to a decline in detections in the northern hemisphere, while some countries in the southern hemisphere reported an increase in influenza detections in recent weeks.
Most influenza activity was very low in North America, the Caribbean, and Central American countries.
In the tropical countries of South America, influenza activity decreased overall during this reporting period, although positivity increased to an extraordinary level in the Plurinational State of Bolivia.
In Europe, overall influenza detections decreased, and influenza positivity from sentinel sites dropped below the epidemic threshold of 10% at the regional level.
Despite continued testing in Central Asia, no influenza detections were reported during this period.
In Northern Africa, no influenza detections were reported.
In Western Asia, influenza activity remained low overall.
In East Asia, influenza activity decreased overall, although detections of mainly influenza A(H1N1)pdm09 continued to increase in Hong Kong Special Administrative Region, China.
A slight increase in influenza detections was reported in the Republic of Korea.
Other influenza and vaccine news for the upcoming 2023-2024 flu season is posted at Precision Vaccinations.
Clover Biopharmaceuticals, Ltd. today announced that it had established a commercial partnership with Keyuan Xinhai Medical Products Trading Co. Ltd. as it prepares for the commercial launch of AdimFlu-S (QIS), the only imported quadrivalent seasonal influenza vaccine approved for use in individuals aged three years and older in China.
“We are thrilled to partner with Kyuan Trade, which has a large and talented commercial team, a broad distribution network that extends across 31 provinces, municipalities, and autonomous regions, and an excellent modern logistics network,” said Joshua Liang, Chief Executive Officer, and Executive Director of Clover, in a press release on May 23, 2023.
Clover expects to launch AdimFlu-S (QIS) later in 2023.
China's seasonal influenza vaccine market has been growing by approximately 30% annually.
Moreover, demand in China continues to shift from trivalent to quadrivalent seasonal influenza vaccine options, which accounted for the majority of doses (70%) in 20222.
The WHO published Influenza Update N° 445 on May 15, 2023, stating:
- In Central Asia, no influenza detections were reported during this period despite continued testing.
- In Western Asia, influenza activity remained low overall, with detections of all seasonal influenza subtypes.
- In East Asia, influenza activity decreased overall, although detections of mainly influenza A(H1N1)pdm09 continued to increase in Hong Kong Special Administrative Region, China.
- A slight increase in influenza detections was reported in the Republic of Korea.
Flu shot news of the 2023-2024 influenza season is posted by Precision Vaccinations.
The U.S. Food and Drug Administration today approved Entasis Therapeutics's Xacduro, an intravenous infusion treatment for hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by susceptible strains of bacteria called Acinetobacter baumannii-calcoaceticus complex, for adult patients 18 years of age and older.
"The FDA is dedicated to supporting the development of safe and effective treatment options for infections caused by difficult-to-treat bacteria like Acinetobacter baumannii-calcoaceticus complex," said Peter Kim, M.D., M.S., director of the Division of Anti-Infectives in the FDA's Center for Drug Evaluation and Research, in a press release on May 23, 2023.
"Today's approval helps address a high unmet medical need by providing an additional treatment option for some of the sickest patients in our nation's hospitals."
According to the World Health Organization, Acinetobacter species top the list of critical bacterial pathogens that pose the greatest threat to human health.
Acinetobacter baumannii-calcoaceticus complex (hereafter referred to as A. baumannii) includes four species of bacteria in the Acinetobacter family.
These bacteria can cause infections in various body parts, occurring most frequently in healthcare settings and predominantly causing pneumonia.
A. baumannii can become highly resistant to multiple antibacterial drugs, and current treatment options for drug-resistant A. baumannii are limited.
Xacduro consists of sulbactam, a drug structurally related to penicillin, and durlobactam.
Sulbactam kills A. baumannii, whereas durlobactam protects sulbactam from being degraded by enzymes that may be produced by A. baumannii.
Previously, the FDA granted Xacduro Fast Track, Qualified Infectious Disease Product, and Priority Review designations for this application.
Additional pneumonia and influenza vaccine news is posted by Precision Vaccinations.

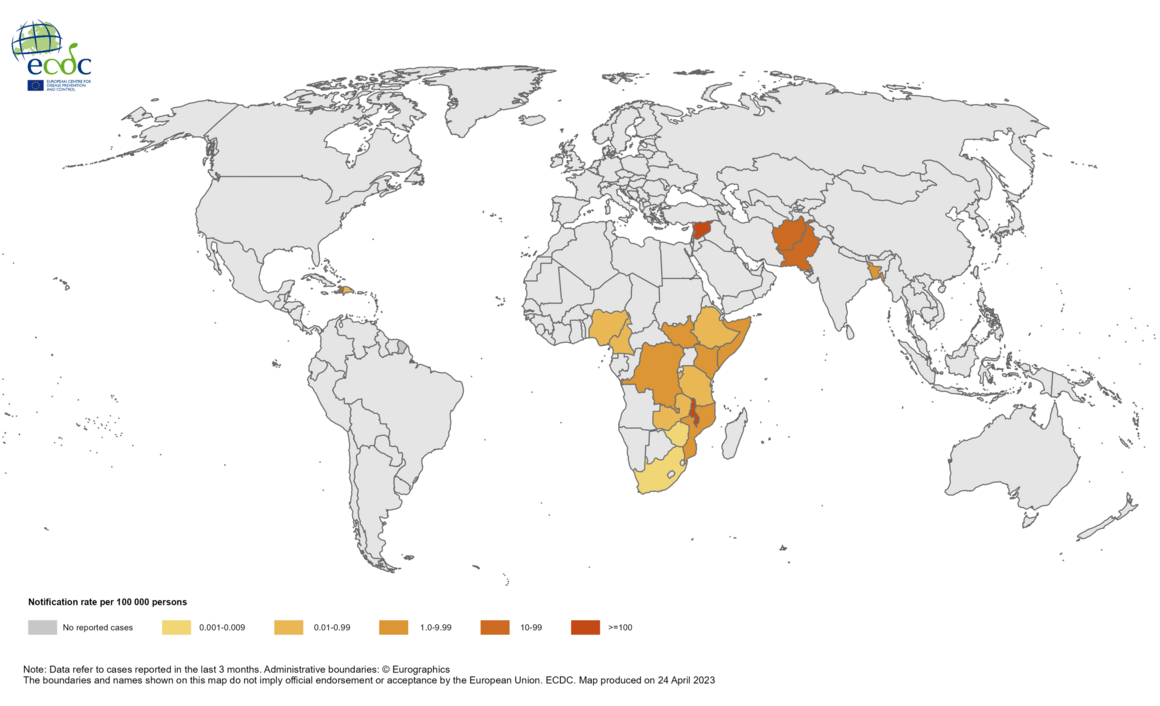
According to Reuters, on May 22, 2023, South Africans blamed Pretoria's Gauteng province government for failing to provide clean water as cholera-related fatalities reached 15.
The Gauteng Department of Health (GDoH) informed the public of the recent outbreak presenting with gastrointestinal symptoms in Hammanskraal, which is located north of Pretoria.
The first cholera cases in this outbreak were imported or import-related cases following travel to Malawi. All subsequent cases acquired infection locally and are classified as indigenous cases.
Other African countries currently experiencing cholera outbreaks include Mozambique, Zambia, and Zimbabwe.
The World Health Organization (WHO) says cholera outbreaks are ongoing in 24 countries.
As of May 23, 2023, there are WHO-approved cholera vaccines such as DUKORAL®; however, due to production constraints, supply is very limited.
Icosavax, Inc. today announced positive topline interim results from its Phase 1 clinical trial of IVX-A12 against the respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) in older adults.
IVX-A12 comprises IVX-121, Icosavax’s RSV prefusion F protein VLP vaccine candidate, and IVX-241, Icosavax’s hMPV prefusion F protein VLP vaccine candidate.
“IVX-A12 is a potential first-in-class combination vaccine candidate designed to address an unmet medical need in older adults, and we believe these interim data for hMPV in an older adult population also break new ground in the field. Furthermore, as has been seen previously with prefusion F antigen approaches for RSV, we expect that a combination vaccine displaying prefusion F antigens for both hMPV and RSV, on our VLP technology, may translate into significant protection against two leading causes of pneumonia. Therefore, we plan to expeditiously proceed towards a Phase 2 trial for IVX-A12 in mid-2023 in older adults as we pursue our goal of developing a broader viral respiratory vaccine,” said Adam Simpson, Chief Executive Officer of Icosavax, in a press release on May 22, 2023.
In this Phase 1 trial, IVX-A12 induced robust immune responses against RSV and hMPV at Day 28 in older adults across dosage levels and with and without adjuvant.
When administered in combination, there was no evidence of immune interference between RSV and hMPV VLPs.
The IVX-121 (RSV) component of IVX-A12 (RSV/hMPV) previously demonstrated positive immunogenicity and tolerability results in a Phase 1/1b study, and a subset of these Phase 1b older adult subjects continue to be followed.
In December 2022, Icosavax reported positive durability data at six months, with twelve-month immunogenicity data expected in mid-2023.
Discovered in 2001, HMPV is in the Pneumoviridae family along with RSV, says the U.S. CDC.
Broader use of molecular diagnostic testing has increased the identification and awareness of HMPV as an important cause of upper and lower respiratory infections.
Other RSV vaccine candidate news is posted by Precision Vaccinations.

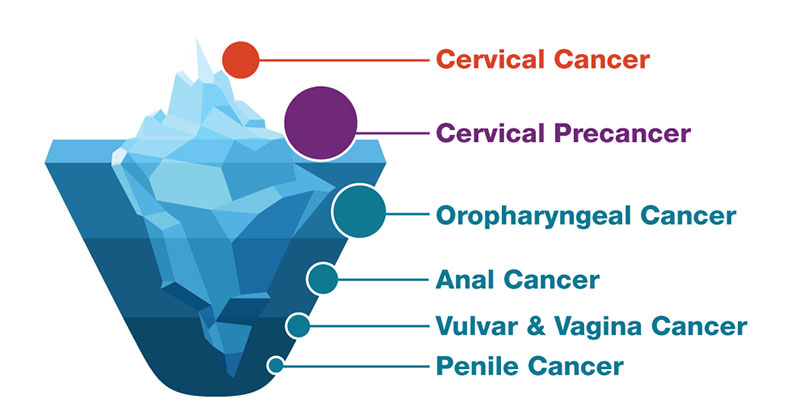
Why do some parents resist offering cancer prevention vaccines to their adolescents? These effective vaccines have significantly reduced cervical cancer over decades.
HPV vaccination could prevent more than 90% of cancers caused by Human Papillomavirus (HPV) from developing.
A new study published today by the American Academy of Pediatrics found parents cited vaccine safety as a leading reason for not intending to vaccinate their adolescent children against HPV has increased over time.
Overall, parental HPV vaccine hesitancy decreased by 5.5% annually between 2010 and 2012 and remained stable for nine years from 2012 through 2020.
The five most frequently cited reasons for not intending to vaccinate included “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
The proportion of parents citing “safety or side effects” as a reason for vaccine hesitancy increased significantly by 15.6% annually from 2010 to 2018.
The proportion of parents citing “not recommended,” “lack of knowledge,” or “child not sexually active” as reasons for vaccine hesitancy decreased significantly by 6.8%, 9.9%, and 5.9%, respectively, per year.
And no significant changes were observed for parents citing “not necessary.”
The good news is by 2020, about 75% of adolescents had received at least one HPV vaccine dose, but only 59% completed the vaccination series.
The U.S. CDC updated its recommended HPV vaccination schedule in 2023.
Precision Vaccinations post other sexually transmitted disease vaccine news.
According to Paraguay's National Animal Health and Quality Service President, Dr. José Carlos Martin, on May 22, 2023, three confirmed outbreaks of avian influenza (bird flu) in the Chaco region occurred, while two others were still under investigation.
MercoPress, a news agency based in Uruguay, reported bird flu was registered in home-breeding establishments with open-air sheds and backyard poultry, in Mariscal Estigarribia, Colonia Neuland, and Filadelfia, in the department of Boquerón.
On May 18, 2023, the Pan American Health Organization announced bird flu outbreaks are mainly occurring in areas along the Pacific flyway and that outbreaks have occurred in 15 countries in Latin America and the Caribbean, which it said is unprecedented.
In North America, Canada and the United States have been battling the virus since early 2022 and have reported it in wild birds, poultry, and mammals.
Additional bird flu outbreak news regarding mammals and people is posted at Precision Vaccinations.