Search API
The U.K. Health Security Agency (UKHSA) today published an analysis focused on the resurgence of mpox in England.
As of May 24, 2023, the UKHSA reported 20 new cases of mpox.
Of these, 19 were in England (10 cases were presumed to have acquired mpox in the U.K., eight were acquired outside the U.K., and one was waiting for classification), and one was in Scotland.
The UKHSA did not disclose the vaccination status of the mpox cases.
During 2022, there were 3,732 confirmed and highly probable mpox cases reported in the U.K. Of these, 3,553 were in England.
Before 2022, cases diagnosed in the U.K. had been imported from countries where mpox is endemic or contacts with documented epidemiological links to imported cases. Between 2018 and 2021, there were 7 cases of mpox in the U.K.
Anyone eligible for mpox vaccination in the U.K. is urged to come forward for the vaccine in the coming months to protect themselves before the summer of 2023.
Steve Russell, NHS director of vaccinations and screening, stated in March 2023, "There is still time to get your first and second doses if you haven't already, which will provide long-term protection against the virus and any possible future outbreaks, so please do book an appointment while the offer is available on the NHS."
Bavarian Nordic's JYNNEOS® (MVA-BN) vaccine is offered in the U.K. as well as in the U.S.
Last week, the U.S. Centers for Disease Control and Prevention confirmed a cluster of mpox infections in mostly vaccinated men in the Chicago, Illinois area.
Mpox is a sexually transmitted disease, similar to HPV and hepatitis, that is vaccine-preventable.

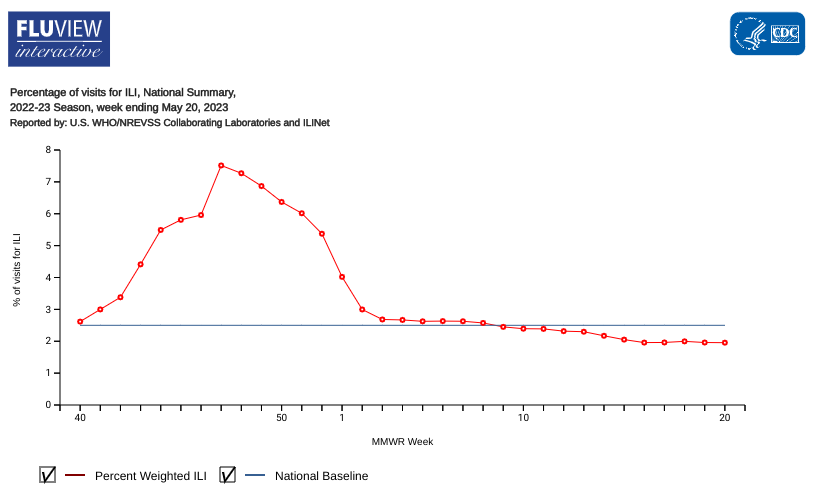
The U.S. Centers for Disease Control and Prevention (CDC) today announced week #20 is the last full version of FluView for the 2022-2023 influenza season.
Starting with week #21, an abbreviated summer version of FluView will be published. The full version is expected to resume for week #40 of 2023, which is the start of the 2023-2024 influenza season.
As of May 26, 2023, seasonal influenza activity remains low nationally, with outpatient respiratory illness below baseline in eight of 10 HHS regions.
Unfortunately, 154 pediatric flu fatalities have been reported so far this season.
The good news is the majority of influenza viruses tested are in the same genetic subclade and antigenically similar to the influenza viruses included in this season’s influenza vaccine.
The CDC continues to recommend that everyone ages six months and older get an annual flu shot as long as flu activity continues in their area, or intend to visit a country where the local flu season is accelerating.
Various influenza vaccines remain available at health clinics and pharmacies in the U.S.
Are you an international traveler looking for an undiscovered, safe destination to visit? According to the U.S. Department of State, the Republic of the Marshall Islands could be an option.
With about six thousand annual visitors in 2019, these Pacific Islands are undiscovered.
This U.S. Territory includes 29 atolls and over 1,200 islands, famous for its numerous shipwrecks.
On May 23, 2023, the State Department's Level 1 travel advisory confirmed without COVID-19 restrictions, visitors to this Oceanic retreat should exercise standard precautions.
Should Americans need assistance, the U.S. Embassy in the Marshall Islands is located at Mejen Weto, Ocean Side, Majuro, Marshall Islands.
However, non-emergency consular services were recently suspended indefinitely. For questions and inquiries, call 247- 4011 or email [email protected].
From a health perspective, there was a shortage of necessary medical supplies and medications in April 2023.
And the U.S. CDC suggests various routine vaccinations before visiting the Marshall Islands.

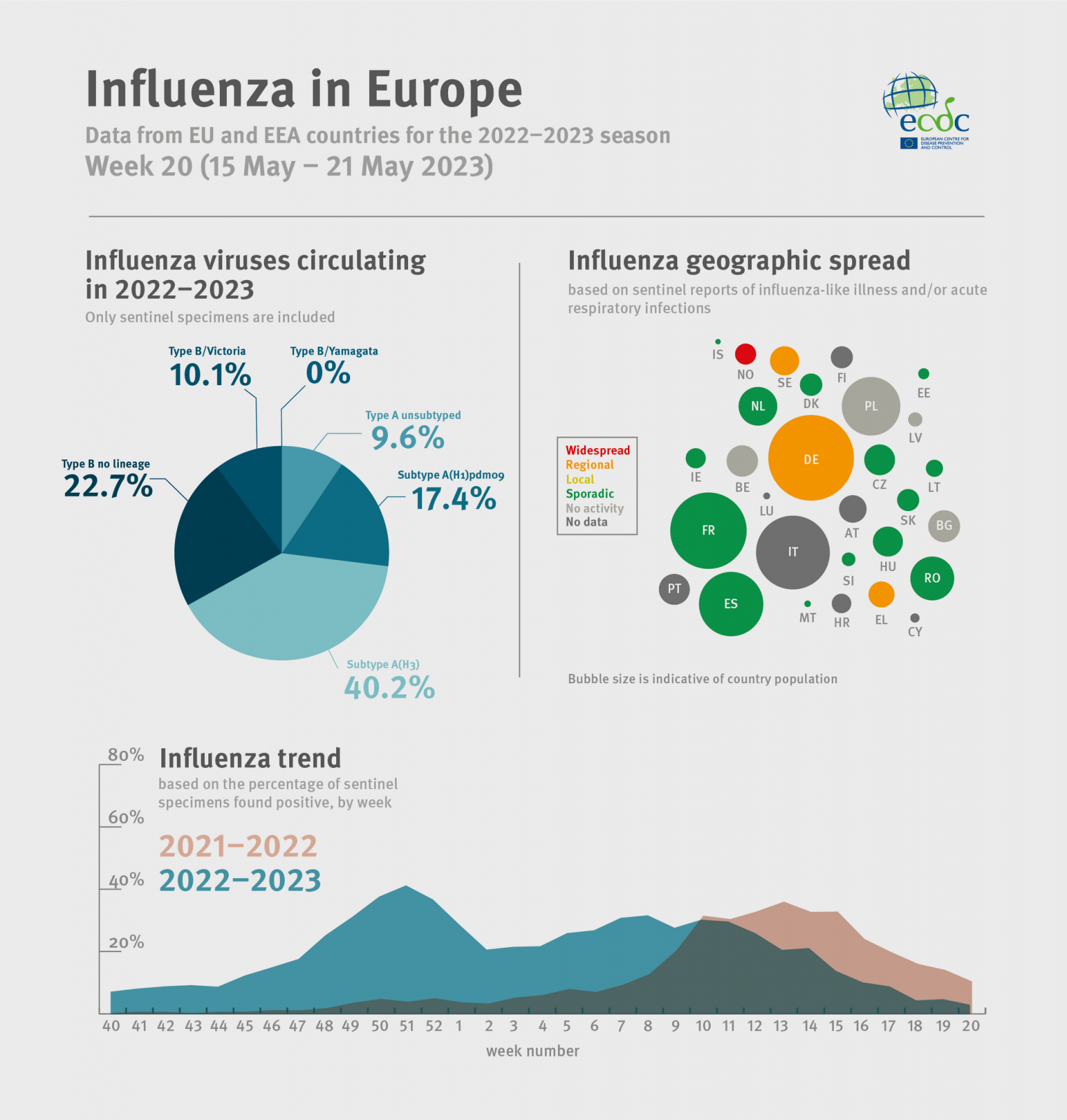
The ECDC and the WHO Regional Office for Europe jointly reported influenza transmissions have significantly decreased.
As of May 21, 2023, the percentage of all sentinel primary care specimens from patients presenting with symptoms that tested positive for an influenza virus decreased to 2% from 4% in the previous week, which is below the epidemic threshold set at 10%.
And 15 of 39 countries or areas reported low intensity; there were no reports of medium or higher intensity, with only 2 of 38 countries across the Region reporting widespread activity.
Both the ECDC and WHO suggest international travelers speak with a healthcare provider about flu shot options for the forthcoming 2023-2023 influenza season.
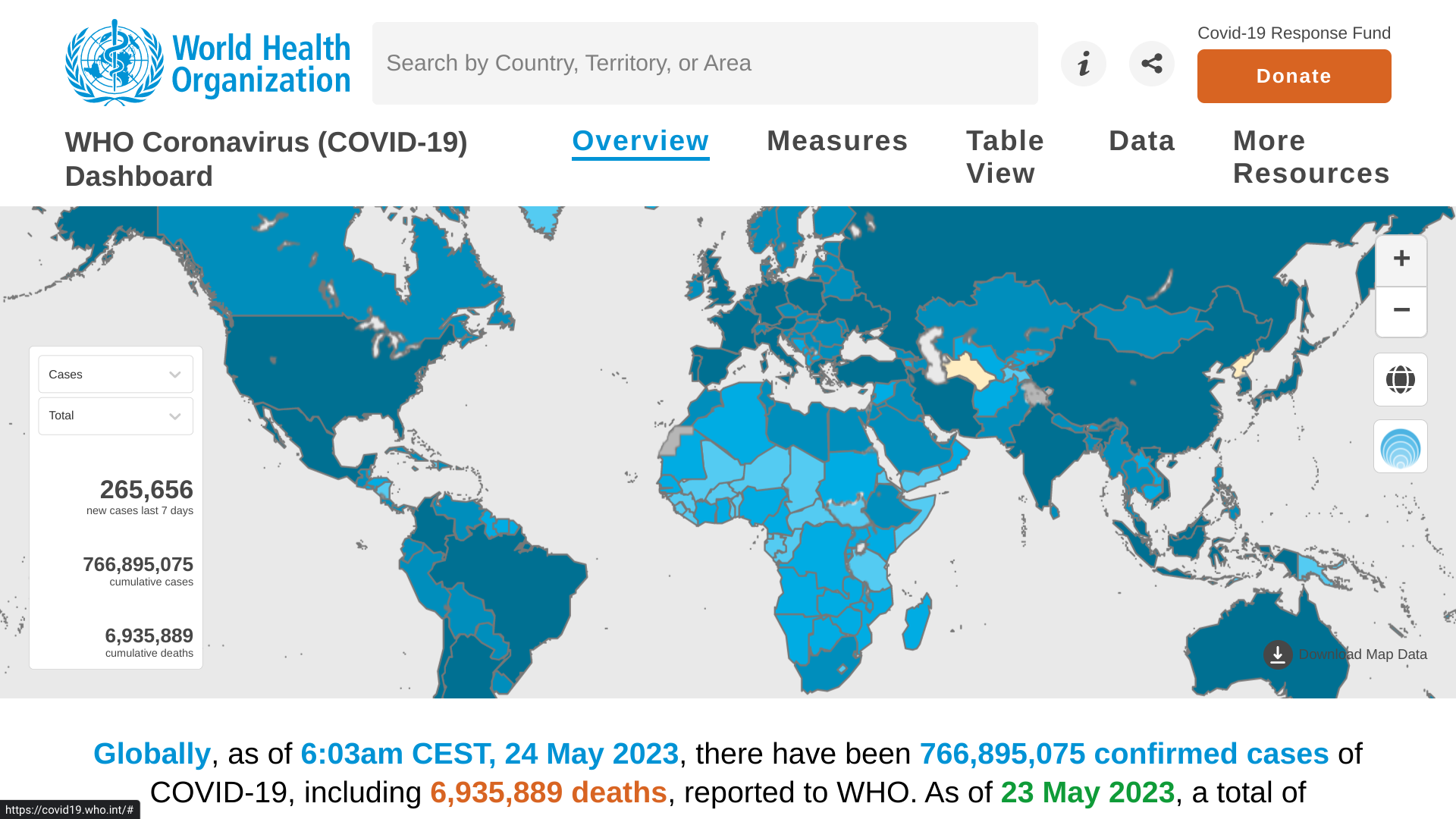
The WHO's Weekly Epidemiological Update #144 provides an overview of the global, regional, and country-level COVID-19 cases and related fatalities.
As of May 25, 2023, nearly 2.3 million new cases and almost 15,000 fatalities were reported in the last 28 days, a decrease of 21% and 17%, respectively, compared to the previous 28 days.
The global situation is mixed at the regional level, with increases in reported cases seen in the WHO African and Western Pacific Regions and increases in fatalities in the African, the Americas, South-East Asia, and Western Pacific Regions.
Disaggregated data are still accessible on the WHO COVID-19 dashboard, where the entire dataset is available for download.
Puerto Rico's Department of Health Arboviral Disease recently reported increased dengue and Zika cases.
As of May 25, 2023, the data for week #19 indicates 305 dengue and 25 Zika probable cases so far this year.
While no Zika vaccines are available, Puerto Rico is evaluating the Dengvaxia® vaccine in the greater San Juan area.
The U.S. CDC has scheduled a vaccine committee to discuss Puerto Rico's test results and review the new QDENGA® vaccine.
Qdenga has been approved for use in Argentina, Brazil, Indonesia, the European Union, and the U.K. as of May 2023.

Biofabri and IAVI recently announced signing an agreement for the end-to-end development of tuberculosis (TB) vaccine candidate MTBVAC. This agreement provides a framework for the future collaboration that the partners first announced in 2021.
After securing sufficient funding, IAVI plans to begin an efficacy clinical trial in 2024.
MTBVAC is a highly promising vaccine candidate that has the potential to be used as an alternative to BCG vaccination in infants and for the prevention of TB disease in adolescents and adults.
"The world urgently needs a new, effective vaccine that can prevent TB disease in adults, adolescents, and infants," said Dr. Mark Feinberg, president, and CEO of IAVI, in a press release on May 17, 2023.
"We are honored to work with Biofabri and our other collaborators to advance MTBVAC."
"In addition, we are actively seeking the support of global health funders and other partners, public and private, to ensure that this promising vaccine candidate has the potential to be part of a solution to ending the TB epidemic."
Should MTBVAC be safe and efficacious, Biofabri will ensure that the TB vaccine is manufactured and supplied in sufficient quantities globally and is accessible at affordable prices in low- and middle-income countries.
Precision Vaccinations post other TB vaccine and outbreak news.

The U.S. Food and Drug Administration (FDA) today approved Pfizer Inc.'s oral antiviral Paxlovid™ for treating mild-to-moderate COVID-19 in adults at high risk for progression to severe COVID-19, including hospitalization or death.
Paxlovid is the fourth drug, but the first oral antiviral approved to treat COVID-19 in adults.
Paxlovid manufactured and packaged under the emergency use authorization (EUA) and distributed by the U.S. Department of Health and Human Services will continue to be available to ensure continued access for adults, as well as treatment of eligible children ages 12-18 who are not covered by the FDA's approval on May 25, 2023.
"While the pandemic has been challenging for all of us, we have made great progress mitigating the impact of COVID-19 on our lives," said Patrizia Cavazzoni, M.D., director for the FDA's Center for Drug Evaluation and Research, in today's press release.
"Today's approval demonstrates that Paxlovid has met the agency's rigorous standards for safety and effectiveness and that it remains an important treatment option for people at high risk for progression to severe COVID-19, including those with prior immunity."
"The FDA remains committed to working with sponsors to facilitate the development of new prevention and treatment options for COVID-19."
Paxlovid is not approved or authorized for use as a pre-exposure or post-exposure prophylaxis to prevent COVID-19.


